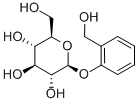
D-(-)-Salicin synthesis
- Product Name:D-(-)-Salicin
- CAS Number:138-52-3
- Molecular formula:C13H18O7
- Molecular Weight:286.28
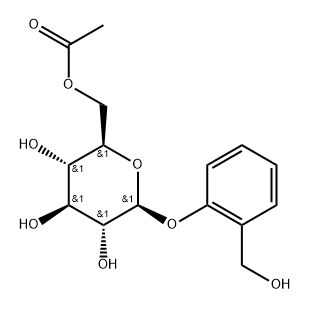
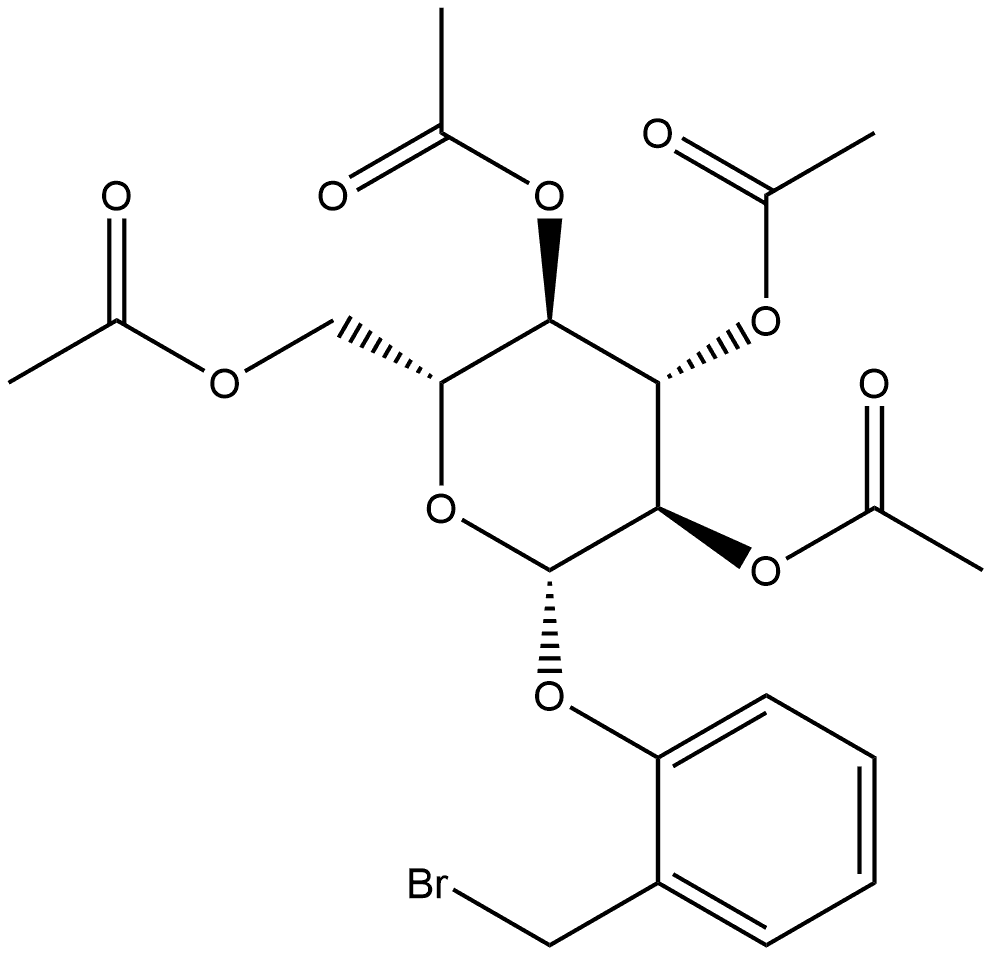
![2-[(Acetyloxy)methyl]phenyl β-D-glucopyranoside 2,3,4,6-tetraacetate](/CAS/GIF/16643-37-1.gif)
16643-37-1

138-52-3
GENERAL METHODS: The compound (CAS:16643-37-1) was used as raw material and mixed with the acceptor ArOH (3.0 eq.) in anhydrous DCM (0.1 M), to which was added freshly prepared BF3-OEt2 solution (1.0 eq., 1 M, in anhydrous DCM). The reaction mixture was stirred at room temperature for 1 h. Subsequently, the progress of the reaction was monitored by TLC (30:70; EtOAc: hexane). Upon completion of the reaction, the reaction was quenched by the addition of NaHCO3 (aqueous), the mixture was extracted with DCM, the organic phase was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuum. The α/β ratio of the crude product was analyzed by 1H NMR. The residue was dissolved in anhydrous methanol (0.3 M) and freshly prepared MeONa solution (0.25 equiv., 0.5 M methanol solution) was added and stirred for 1 h at room temperature. The reaction mixture was neutralized with Amberlite IR-120H+ , filtered and concentrated in vacuum to give a white solid. Purification by fast column chromatography (10:90; MeOH:DCM) removes excess ArOH to give β-D isoheads, which are finally recrystallized in hot ethanol.
![2-[(Acetyloxy)methyl]phenyl β-D-glucopyranoside 2,3,4,6-tetraacetate](/CAS/GIF/16643-37-1.gif)
16643-37-1
0 suppliers
inquiry

138-52-3
520 suppliers
$6.00/1g
Yield:138-52-3 80 mg
Reaction Conditions:
with sodium methylate in methanol at 20; for 1 h;Inert atmosphere;
Steps:
Glucosides 24-28 General procedure
General procedure: To a solution 2 and the acceptor ArOH (3.0 equiv) in dry DCM (0.1 M) was added afreshly prepared solution of BF3?OEt2 (1.0 equiv., 1M in dry DCM). The solution wasstirred 1 h and followed by TLC (30:70; EtOAc : hexanes). A solution of NaHCO3(aq) wasadded and the mixture was extracted with DCM, washed with brine, dried over Na2SO4,filtered and concentrated in vacuo. The α/β ratio was measured by 1H NMR on the crude.The residue was treated with a freshly prepared solution MeONa (0.25 equiv., 0.5 M inmethanol) in dry methanol (0.3 M). The solution was stirred at r.t. for 1 h andneutralized with Amberlite IR-120 H+, filtered and concentrated in vacuo to give a whitesolid. Quick flash column chromatography (10:90; MeOH:DCM) removed excess of ArOHand led to the β-D anomer, which was recrystallized in hot ethanol.
References:
St-Pierre, Gabrielle;Dafik, Laila;Klegraf, Ellen;Hanessian, Stephen [Synthesis,2016,vol. 48,# 20,p. 3575 - 3588] Location in patent:supporting information
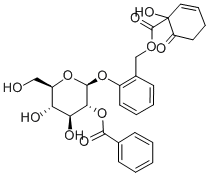
19764-02-4
2 suppliers
inquiry

138-52-3
520 suppliers
$6.00/1g
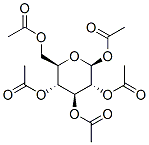
29836-40-6
16 suppliers
inquiry

138-52-3
520 suppliers
$6.00/1g
604-69-3
398 suppliers
$10.00/25g

138-52-3
520 suppliers
$6.00/1g
60523-66-2
0 suppliers
inquiry

138-52-3
520 suppliers
$6.00/1g