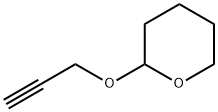
TETRAHYDRO-2-(2-PROPYNYLOXY)-2H-PYRAN synthesis
- Product Name:TETRAHYDRO-2-(2-PROPYNYLOXY)-2H-PYRAN
- CAS Number:6089-04-9
- Molecular formula:C8H12O2
- Molecular Weight:140.18
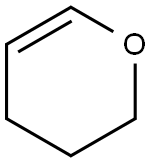
110-87-2

107-19-7

6089-04-9
General procedure for the synthesis of O-THP propargyl alcohol (Compound 18) from 3,4-dihydro-2H-pyran and 2-propyn-1-ol: Compound 18 was prepared according to literature procedure. To a solution of propargyl alcohol 17 (2.6 mL, 44.6 mmol) and p-toluenesulfonic acid monohydrate (0.09 g, 0.5 mmol) in dichloromethane (45 mL) was added dropwise 3,4-dihydro-2H-pyran (4.3 mL, 46.8 mmol) at 0 °C. The reaction mixture was stirred at 0 °C for 5 min before slowly warming up to room temperature and continued stirring for 1 h. The reaction mixture was then purified with aqueous solution of saturated 2H-pyran (4.3 mL). Subsequently, the reaction mixture was washed with saturated sodium bicarbonate solution (30 mL) and the aqueous layer was extracted with dichloromethane (45 mL). The organic layers were combined, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give compound 18 (6.1 g, 97.2% yield) as a yellow oil. Thin layer chromatography (TLC) Rf = 0.30 (hexane/ether, 95:5). 1H NMR (400 MHz, CDCl3): δ = 1.50-1.67 (m, 4H), 1.70-1.89 (m, 2H), 2.41 (t, J = 2.4 Hz, 1H), 3.52-3.57 (m, 1H), 3.82-3.87 (m , 1H), 4.27 (ddd, J = 2.4 Hz, 11.3 Hz, 15.8 Hz, 2H), 4.83 (t, J = 3.4 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ = 19.0, 25.3, 30.2, 53.9, 61.9, 74.0, 79.7, 96.8. mass spectra (MS-CI): m/z calculated values. ): m/z calculated value C8H12O2 [M + H]+, 141; measured value: 141. The synthesis of this compound has been reported in the literature.
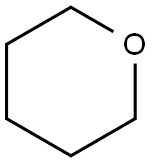
142-68-7
186 suppliers
$13.00/25mL

107-19-7
0 suppliers
$12.00/25g

6089-04-9
125 suppliers
$13.43/1gm:
Yield:6089-04-9 90%
Reaction Conditions:
with toluene-4-sulfonamide in dichloromethane at 20; for 24 h;Cooling with ice;Reagent/catalyst;
Steps:
1-2 Example 1
Add 0.3568mol propynol, 150mL dichloromethane, 0.03568mol p-toluenesulfonamide, cooling in an ice bath, slowly drip 42.02g of dihydropyran, after the addition is complete, stir at room temperature for 24h, after the reaction was completed, saturated aqueous sodium bicarbonate solution was added to quench the reaction, the organic phase was extracted with dichloromethane, washed with water, brine, after drying with anhydrous sodium sulfate and purifying by column chromatography, compound 2 was obtained with a yield of 90%.
References:
CN112661725, 2021, A Location in patent:Paragraph 0034-0037

110-87-2
543 suppliers
$10.00/1g

107-19-7
0 suppliers
$12.00/25g

6089-04-9
125 suppliers
$13.43/1gm:

110-87-2
543 suppliers
$10.00/1g
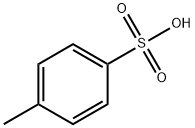
104-15-4
764 suppliers
$133.00/500g

6089-04-9
125 suppliers
$13.43/1gm:

110-87-2
543 suppliers
$10.00/1g

927-74-2
426 suppliers
$6.00/5g

6089-04-9
125 suppliers
$13.43/1gm:
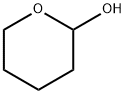
694-54-2
86 suppliers
$16.00/1g
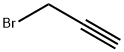
106-96-7
336 suppliers
$30.00/25g

6089-04-9
125 suppliers
$13.43/1gm: