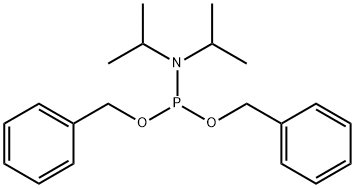
DIBENZYL DIISOPROPYLPHOSPHORAMIDITE synthesis
- Product Name:DIBENZYL DIISOPROPYLPHOSPHORAMIDITE
- CAS Number:108549-23-1
- Molecular formula:C20H28NO2P
- Molecular Weight:345.42
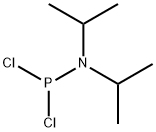
921-26-6

100-51-6

108549-23-1
General procedure for the synthesis of diphenyl N,N'-diisopropylphosphoramidite from dichloro-N,N-diisopropylphosphoramidite and benzyl alcohol: 42 g (207.92 mmol) of dichloro-N,N-diisopropylphosphoramidite was placed in a 500 mL round-bottomed flask, and 300 mL of anhydrous tetrahydrofuran was added, and dissolved by stirring. Subsequently 66 mL (455.44 mmol, 46 g) of anhydrous triethylamine was added. The flask was cooled to 0°C in an ice-salt bath and 45 mL (416.67 mmol, 45 g) of anhydrous benzyl alcohol was added slowly and dropwise under nitrogen protection, keeping the reaction temperature close to 0°C. Upon completion of the reaction, the suspension was added dropwise and the formation of a white solid was observed. The reaction mixture was removed from the ice bath, returned to room temperature and stirring was continued for 3 hours. After stopping stirring, it was allowed to stand for 10 minutes for filtration and the solid was washed with tetrahydrofuran. The filtrate was collected and the solvent was removed by rotary evaporation to give the crude product. Without column chromatographic purification, 54.0 g of the oily product was obtained directly in 75.3% yield.

921-26-6
83 suppliers
$80.00/1g

100-51-6
1432 suppliers
$5.00/100g

108549-23-1
207 suppliers
$5.00/1g
Yield:108549-23-1 75.3%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0; for 1 h;Inert atmosphere;Cooling with ice;
Steps:
viii (νiii) The synthesis of (i-Pr)2NP(OBn)2 (3-7)
Take compound 3-9, 42g (207.92mmo1) was placed in a 500mL round bottomed flask, was added 300mL of anhydrous tetrahydrofuran, 66mL (455.44mmol, 46g) of anhydrous triethylamine were dissolved with stirring. When the flask was placed in an ice-salt bath until the system temperature was lowered to 0 the right, under nitrogen was added dropwise over anhydrous benzyl alcohol 45mL (416.67mmol, 45g), was added dropwise maintaining temperature of the system is always near 0 , IH complete after the suspension was added dropwise to give a white solid containing the ice bath was removed to room temperature, stirring was continued at room temperature for 3h stirring was stopped to stand 10min, filtered off with suction, washed solid was tetrahydrofuran, the filtrate was collected, rotary evaporated to give tetrahydrofuran was no liquid column liquid chromatography to give an oil 54.0g, a yield of 75.3%.
References:
Nankai University;Xi Zhen;Song Fanbo;Wang Ting;Cui Quanbin;Zhou Kai;Liu Jianbing CN102977145, 2017, B Location in patent:Paragraph 0196; 0197
108-18-9
385 suppliers
$10.00/1g

108549-23-1
207 suppliers
$5.00/1g

108-18-9
385 suppliers
$10.00/1g

100-51-6
1432 suppliers
$5.00/100g

108549-23-1
207 suppliers
$5.00/1g
108549-21-9
39 suppliers
$106.00/1G

100-51-6
1432 suppliers
$5.00/100g

108549-23-1
207 suppliers
$5.00/1g
76101-29-6
21 suppliers
inquiry

108549-23-1
207 suppliers
$5.00/1g