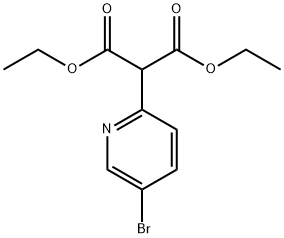
DIETHYL(5-BROMOPYRIDIN-2-YL)MALONATE synthesis
- Product Name:DIETHYL(5-BROMOPYRIDIN-2-YL)MALONATE
- CAS Number:1215098-80-8
- Molecular formula:C12H14BrNO4
- Molecular Weight:316.15
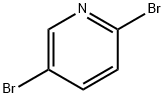
223463-13-6
387 suppliers
$5.00/1g

105-53-3
793 suppliers
$5.00/25g

1215098-80-8
11 suppliers
inquiry
Yield: 97%
Reaction Conditions:
with 2-Picolinic acid;copper(l) iodide;caesium carbonate in 1,4-dioxane at 70;
Steps:
xxvii.a Diethyl 2-(5-bromopyridin-2-yl)malonate 166
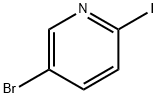
To a solution of 5-bromo-2-iodopyridine (18.0 g, 63.4 mmol) in 1 ,4-dioxane (100 mL) was added diethyl malonate (20.3 g, 126.8 mmol), Cs2C03 (62.0 g, 190.2 mmol), Cul (1 .2 g, 6.3 mmol) and picolinic acid (1 .6 g, 12.7 mmol). The resulting mixture was heated to 70 °C and stirred overnight. Water (100 mL) and EtOAc (100 mL) were added and the aqueous layer extracted with EtOAc (3 x 50 mL). The combined organic extracts were washed with water (3 x 50 mL) and brine (3 x 50 mL), dried (Na2SO.t) and concentrated. The crude product was purified by silica gel chromatography (ethyl acetate/petroleum ether = 1 /20) to give the title compound as yellow oil (19.6 g, 97%). LCMS-C: RT 2.49 min; m/z 316.0, 318.0 [M+H]+
References:
CTXT PTY LTD;BERGMAN, Ylva Elisabet;FOITZIK, Richard Charles;MORROW, Benjamin Joseph;CAMERINO, Michelle Ang;WALKER, Scott Raymond;LAGIAKOS, H. Rachel;FEUTRILL, John;STEVENSON, Graeme Irvine;STUPPLE, Paul Anthony WO2016/34673, 2016, A1 Location in patent:Page/Page column 100