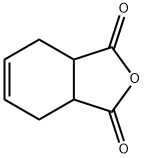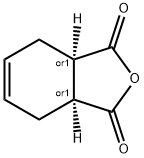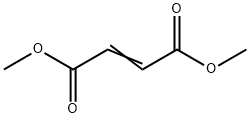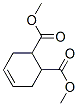
dimethyl cyclohex-3-ene-1,6-dicarboxylate synthesis
- Product Name:dimethyl cyclohex-3-ene-1,6-dicarboxylate
- CAS Number:7500-55-2
- Molecular formula:C10H14O4
- Molecular Weight:198.22
Yield:7500-55-2 92 %
Reaction Conditions:
with sulfuric acidReflux;
Steps:
9 Example 9: Preparation of Compound II (8-oxabicyclo[4.3.0]-3-nonene)
Alcoholysis and esterification of tetrahydrophthalic anhydride with methanol to obtain dimethyl cyclohexene dicarboxylate, then homogeneous hydrogenation of dimethyl cyclohexene dicarboxylate to retain the double bond, to obtain cyclohexene dimethanol, and then dehydration to obtain Intermediate 8-oxabicyclo[4.3.0]-3-nonene (II).Add 300g of tetrahydrophthalic anhydride powder into a 2L four-neck round bottom flask, add 600g of methanol, start stirring, then slowly drop in 14.5g of concentrated sulfuric acid, start timing when heating to reflux, and distill methanol after half an hour of reflux, every 30 minutes Gas chromatography (GC) analysis, and add an equivalent amount of methanol according to the amount of distilled methanol.Reflux for 2.5h and the reaction is over, the reaction is completely cooled to room temperature, add 15.67g of sodium carbonate with sulfuric acid and other substances, stir to remove unreacted sulfuric acid, remove the solid in the reaction system by suction filtration, and spin the methanol to dry to obtain the crude product. After vacuum distillation, 359.6 g (92% yield, molar yield) of a product with a purity of more than 99% was obtained.
References:
CN115232057,2022,A Location in patent:Paragraph 0192-0195