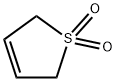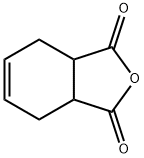
1,2,3,6-Tetrahydrophthalic anhydride synthesis
- Product Name:1,2,3,6-Tetrahydrophthalic anhydride
- CAS Number:85-43-8
- Molecular formula:C8H8O3
- Molecular Weight:152.15
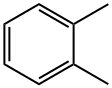
95-47-6
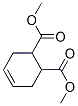
7500-55-2
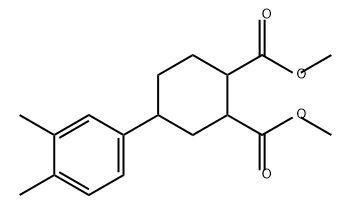
1352221-13-6

85-43-8
2.0 g of lanthanum nitrate hexahydrate was dissolved in 100 mL of deionized water, and the resulting solution was added dropwise to a suspension formed from 5.0 g of H-type Y-zeolite (Tosoh Corporation, HSZ-320H0A, Si/Al ratio = 2.8) with 200 mL of deionized water at room temperature. Subsequently, the mixture was heated at 90 °C for 3 hours. Upon completion of the reaction, the solid product was separated by centrifugation, washed five times with 45 mL of deionized water, and dried at 85 °C overnight. The dried solid was calcined in an air atmosphere at 500 °C for 2 h to obtain the alkylation catalyst (hereinafter referred to as La/HY). The content of La in the catalyst was determined to be 5.6 wt% by ICP analysis. The alkylation reaction was carried out in a SUS autoclave equipped with a 50 mL glass liner. To the glass liner were added 0.10 g of the above La/HY catalyst (100 wt% relative to the unsaturated compounds), 0.16 g of tridecane (internal standard), 0.1 g (0.53 mmol) of methyl 1-cyclohexene-4,5-dicarboxylate (hereinafter referred to as CEDA) as the unsaturated compounds, and 3.0 g (28.30 mmol) of o-xylene as the aromatic compound. The system was sealed under nitrogen atmosphere after three displacements with nitrogen. The reaction was initiated by immersing the autoclave in an oil bath preheated to 180 °C. The reaction was carried out in a water bath. After 2 hours of reaction, the reactor was cooled with water to release the internal gases. The alkylation products 3,4-dimethoxycarbonylcyclohexyl-3',4'-dimethylbenzene (hereinafter referred to as CDMB) and 1-cyclohexene-4,5-dicarboxylic anhydride, etc., in the reaction solution were quantitatively analyzed by gas chromatography with an FID detector as an internal standard method. The results showed that the yield of CDMB was 49.6% and the selectivity was 51.0%.CDMB is a new compound with the following physical properties: boiling point: 166 °C/66 Pa; 1H NMR (500 MHz, CDCl3): δ 1.44-1.48 (m, 1H), 1.69-1.76 (m, 1H), 1.95-2.04 (m, 2H), 2.13-2.16 (m, 1H), 2.22 (s, 3H), 2.24 (s, 3H), 2.33-2.38 (m, 1H), 2.48-2.56 (m, 2H), 3.37-3.96 (m, 1H), 3.71 (s, 3H*2), 6.92 (d, J=7.7 Hz, 1H), 6.96 (s, 1H), 7.06 (d, J=7.7 Hz, 1H) 7.06 (d, J=7.7 Hz, 1H); 13C NMR (500 MHz, CDCl3): δ 19.3, 19.8, 24.5, 33.2, 35.5, 39.0, 42.0, 43.3, 51.8, 123.9, 128.2, 129.7, 134.4, 136.5, 143.4, 173.8, 174.2; IR (d, J=7.7 Hz, 1H). 174.2; IR (neat, cm-1): 3450, 3000, 2950, 2860, 1734.
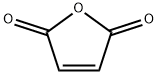
108-31-6
886 suppliers
$13.47/50g
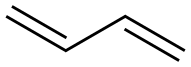
106-99-0
260 suppliers
$80.00/100mL

85-43-8
211 suppliers
$145.00/10g
Yield:85-43-8 98.1%
Reaction Conditions:
at 80 - 110; for 4 h;Large scale;Temperature;
Steps:
1-4 example 2
Maleic anhydride (490 g, 5 mol) was placed in a bubbling reactor, heated to 80 ° C to melt maleic anhydride, and continuously passed from the bottom of the bubbling reactor1,3-butadiene gas,The reaction temperature was controlled to 110 ° C. After 4 hours of reaction, the gas phase was detected, the reaction of the starting material was completed, and the temperature was solidified to obtain 746.3 g of a white solid. The yield was 98.1%.
References:
CN108586398,2018,A Location in patent:Page/Page column 4-6

95-47-6
615 suppliers
$10.00/25g

7500-55-2
5 suppliers
inquiry

1352221-13-6
1 suppliers
inquiry

85-43-8
211 suppliers
$145.00/10g
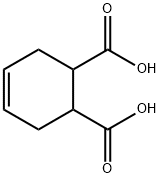
88-98-2
32 suppliers
$164.00/5g

85-43-8
211 suppliers
$145.00/10g

108-31-6
886 suppliers
$13.47/50g
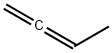
590-19-2
60 suppliers
$225.00/5G

85-43-8
211 suppliers
$145.00/10g