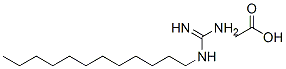
Dodine synthesis
- Product Name:Dodine
- CAS Number:2439-10-3
- Molecular formula:C13H29N3.C2H4O2
- Molecular Weight:287.44

124-22-1
410 suppliers
$5.00/25g
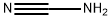
420-04-2
372 suppliers
$15.00/25g
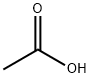
64-19-7
1626 suppliers
$10.00/25ML

2439-10-3
151 suppliers
$25.39/250mg
Yield:-
Reaction Conditions:
Stage #1:n-Dodecylamine;acetic acid in water;butan-1-ol at 60 - 90; for 3 h;
Stage #2:CYANAMID in water;butan-1-ol at 90; for 3 h;
Steps:
Synthesis of laurylguanidinium acetate: 44.1 g of laurylamine (0.238 mol) and 150 ml of n-butanol were initially introduced and dissolved at 60° C. At the elevated temperature, 14.2 g of acetic acid (99.8%, 0.237 mol) were then added. A temperature of 90° C. was then established and, over the course of 3 hours, the cyanamide (11.0 g; 0.262 mol) dissolved in 50 ml of n-butanol was added dropwise. When the addition was complete, the mixture was left to after-react for 3 hours at 90° C. The solvent was then stripped off on a rotary evaporator. For purification, the crude prdduct was slurried in acetone, filtered, and the residue was washed with diethyl ether. Residual amounts of diethyl ether were removed on the rotary evaporator at reduced pressure. This gave a colorless, crystalline powder. Lawylguanidinium acetate: 13C-NMR, 100 MHz, MeOD, 25° C.: δ=179.1 (1C, COOHAcOH), 157.7 (1C, Cguanidinium gr.), 41.2 (1C, CH2), 31.6 (1C, CH2), 29.3 (4C, CH2), 28.9 (2C, CH2), 28.6 (1C, CH2), 26.4 (1C, CH2), 23.2 (1C, CH2),22.3 (1C, CH3AcOH), 13.1 (1C, CH3); MADI-TOF[m/z]: 228 (M+H+)
References:
GOLDSCHMIDT AG US2006/134056, 2006, A1 Location in patent:Page/Page column 6