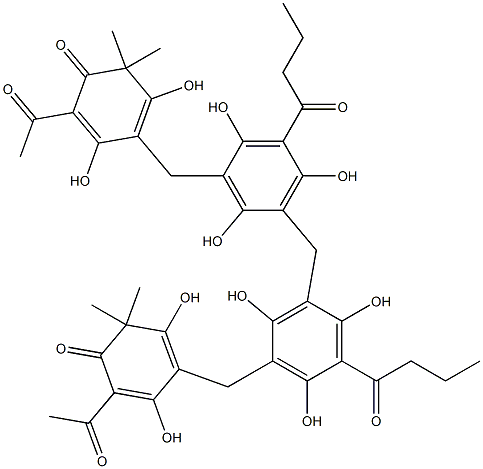
dryocrassin synthesis
- Product Name:dryocrassin
- CAS Number:12777-70-7
- Molecular formula:C43H48O16
- Molecular Weight:820.83
![2,5-Cyclohexadien-1-one, 2-acetyl-3,5-dihydroxy-4,4-dimethyl-6-[[2,4,6-trihydroxy-3-(1-oxobutyl)phenyl]methyl]-](/CAS/20210305/GIF/39007-95-9.gif)
39007-95-9
1 suppliers
inquiry

12777-70-7
119 suppliers
inquiry
Yield: 49%
Reaction Conditions:
with formaldehyd;sulfuric acid in dichloromethane for 3 h;Solvent;
Steps:
4.1.3. General procedure for the preparation of a5, b5 and dryocrassin ABBA
General procedure: To a solution of reactant (50 mg, 0.3 mmol) in DMC (0.3 M) (THF for b5) was added 37% formalin (0.72 mL, 9 mmol) and concentrated sulphuric acid (2.5 mmol) and the reaction mixture was stirred for 3 h (50 °C and 8 h for b5). Saturated sodium bicarbonate was added to neutralize and ethyl acetate was added to extract. Organic layers were combined and then washed with brine, dried with anhydrous Na2SO4, and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to obtain the product. a5 (white powder, 70%): 1H NMR (400 MHz, CDCl3, 25 °C): δ 18.44 (s, 2H), 12.31(s, 2H), 3.31 (s, 2H), 2.73 (s, 6H), 1.54 (s, 6H), 1.47 (s, 6H). 13C NMR (101 MHz, CDCl3, 25 °C): δ 203.3, 199.5, 187.7, 173.6, 110.8, 108.6,44.6, 29.4, 25.4, 24.3, 18.1; IR (KBr) νmax: 2989, 2659, 1639, 1573,1431, 1202, 1207 cm-1; (-)-ESIMS m/z [M-H]-: 403; (-)-HRESIMSm/z [M-H]-: calcd for C21H24O8, 403.1398, found m/z: 403.1402. b5(white powder, 61%): 1H NMR (400 MHz, CDCl3, 25 °C): δ 10.16 (s,2H), 9.75 (br s,2H), 3.72 (s, 2H), 1.73 (q, J=1.73 Hz, 4H), 1.01 (t,J=7.4 Hz, 6H); 13C NMR (101 MHz, CDCl3, 25 °C): δ 207.9, 193.3,169.8, 168.7, 165.1, 105.0, 104.9, 103.6, 45.6, 17.9, 14.8, 14.0; IR(KBr) νmax: 3269; 2963, 1633, 1472, 1186, 1020 cm-1; (-)-ESIMS m/z[M-H]-: 459; (-)-HRESIMS m/z [M-H]-: calcd for C23H24O10,459.129, found m/z: 459.1302. Dryocrassin ABBA (49%, see Supporting information, Table S1 and Scheme S2).
References:
Hou, Bo;Liu, Ze;Yang, Xiao-Bei;Zhu, Wen-Fei;Li, Jin-Yu;Yang, Liu;Reng, Fu-Cai;Lv, Yong-Feng;Hu, Jiang-Miao;Liao, Guo-Yang;Zhou, Jun [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 17,p. 3846 - 3852]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

12777-70-7
119 suppliers
inquiry
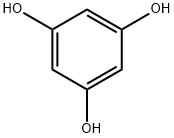
108-73-6
549 suppliers
$13.00/5g

12777-70-7
119 suppliers
inquiry
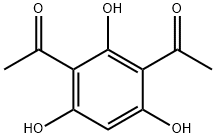
2161-86-6
81 suppliers
$25.00/10 mg

12777-70-7
119 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

12777-70-7
119 suppliers
inquiry