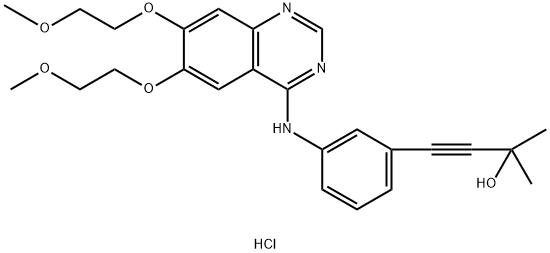
Erlotinib iMpurity 2 synthesis
- Product Name:Erlotinib iMpurity 2
- CAS Number:299912-59-7
- Molecular formula:C25H30ClN3O5
- Molecular Weight:487.98
69088-96-6
111 suppliers
$46.00/250mg
183322-18-1
298 suppliers
$6.00/1g

299912-59-7
15 suppliers
inquiry
Yield:299912-59-7 100%
Reaction Conditions:
in acetonitrile at 25; for 5 h;Inert atmosphere;Reflux;
Steps:
9
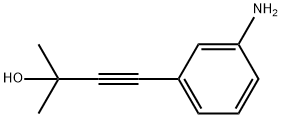
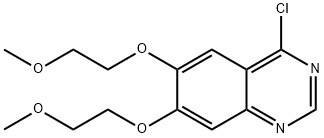
Example 9 - Synthesis of 3-Butyn-2-ol, 4-[3-[[6,7-bis(2-methoxyethoxy)-4-quinazolinyl]amine] phenyl]-2-methyl-, hydrochloride (1:1) of formula (II) - exemplifying the invention.; Synthesis scheme [Show Image] In a 500 ml flask provided with a thermometer there are introduced - in succession at 25°C and under nitrogen atmosphere - 1 5 g of 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline of formula (III), 9.2 g of 4-(3-aminophenyl)-2-methyl-3-butyn-2-ol of formula (IV) and 225 mL of Acetonitrile. The mixture is heated at reflux for 5 hours. Upon completing the reaction the mixture is cooled to 0-5°C and maintained at that temperature under stirring for at least 1 hour. The suspension is filtered and the solid is washed using 15 mL of cold Acetonitrile. The product is dried under vacuum for at least 8 hours obtaining 23.4 g of product as a white solid for a molar yield equivalent to 100%.
References:
EP2433931,2012,A1 Location in patent:Page/Page column 11

50624-25-4
23 suppliers
$130.00/100mg

299912-59-7
15 suppliers
inquiry
585-79-5
30 suppliers
$15.00/1g

299912-59-7
15 suppliers
inquiry
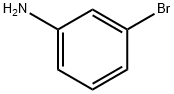
591-19-5
334 suppliers
$10.00/10g

299912-59-7
15 suppliers
inquiry
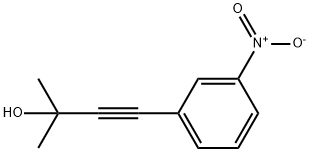
33432-52-9
11 suppliers
inquiry

299912-59-7
15 suppliers
inquiry