Erlotinib synthesis
- Product Name:Erlotinib
- CAS Number:1384975-87-4
- Molecular formula:C13H17NO7
- Molecular Weight:299.28
80407-64-3
63 suppliers
$6.00/100mg

1384975-87-4
14 suppliers
inquiry
Yield:1384975-87-4 64%
Reaction Conditions:
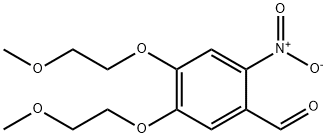
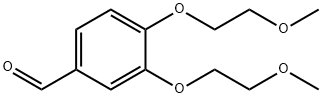
Stage #1: 3,4-bis(2-methoxyethoxy)benzaldehydewith nitric acid in acetic acid at 4; for 1 h;
Stage #2: with sulfuric acid;potassium nitrate in acetic acid at 4 - 20;
Steps:
25.B 4,5-Bis-(2-methoxy-ethoxy)-2-nitro-benzaldehyde
To a solution of 3,4-bis-(2-methoxy-ethoxy)-benzaldehyde (6 g, 23.6 mmol) in acetic acid (30 mL) cooled at 4° C., was added nitric acid (3 mL) (Aldrich). The reaction mixture was stirred for 1 hour. Then, sulfuric acid (3 mL) and potassium nitrate (2.62 g, 26 mmol) (Aldrich) were added to the reaction mixture which was slowly warmed to room temperature and stirred overnight. After completion of the reaction, ammonium hydroxide was added to obtain a pH=10. The reaction mixture was diluted with EtOAc and washed with brine. The combined organic phase was dried over anhydrous sodium sulfate and evaporated. The crude material was purified by column chromatography. The desired compound was eluted with 70% EtOAc. The fractions were evaporated to afford 4,5-bis-(2-methoxy-ethoxy)-2-nitro-benzaldehyde. (Yield 4.5 g, 64%).
References:
US2012/184548,2012,A1 Location in patent:Page/Page column 24
139-85-5
849 suppliers
$5.00/1g

1384975-87-4
14 suppliers
inquiry