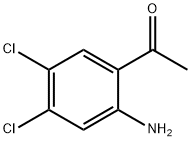
Ethanone,1-(2-amino-4,5-dichlorophenyl)- synthesis
- Product Name:Ethanone,1-(2-amino-4,5-dichlorophenyl)-
- CAS Number:6951-70-8
- Molecular formula:C8H7Cl2NO
- Molecular Weight:204.05
Yield:6951-70-8 6.7 %
Reaction Conditions:
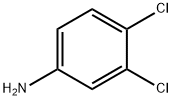
Stage #1: m,p-dichloroaniline;acetonitrilewith aluminum (III) chloride;boron trichloride at 0 - 80;
Stage #2: with hydrogenchloride;water at 0 - 80;
Steps:
2.1 Scheme 1, step 1.1-(2-amino-4,5-dichlorophenyl)ethenone:
To a solution of 3,4-dichloroaniline (2.0 g, 12.4 mmol, 1.0 equiv.) in ACN (20 mL) was added BCl3 (1 M, 13.0 mL, 1.05 eq) at 0 °C. Then AlCl3 (1.81 g, 13.58 mmol, 742 μL, 1.1 equiv.) was added in three portions and the mixture was stirred at 80 °C for 16 hours. The mixture was cooled to 0 °C, and aqueous HCl (4 M, 5 mL) was added and the mixture was stirred at 80 °C for 4 hours. The reaction was cooled to room temperature, extracted with ethyl acetate, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by column chromatography (SiO2, 30:1 petroleum ether: EtOAc) to afford the title compound (170 mg, 6.7% yield) as a white solid. LCMS: calculated for [M+H]+ (C8H7Cl2NO) requires m/z = 204.1, found m/z = 204.1. 1H NMR (400 MHz, DMSO-d6) δ 7.92 (s, 1H), 7.39 (br s, 2H), 7.02 (s, 1H), 2.52 (s, 3H).
References:
WO2022/187203,2022,A1 Location in patent:Paragraph 00124-00126
6635-71-8
0 suppliers
inquiry

6951-70-8
13 suppliers
inquiry