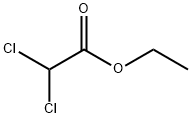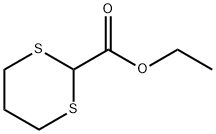
Ethyl 1,3-dithiane-2-carboxylate synthesis
- Product Name:Ethyl 1,3-dithiane-2-carboxylate
- CAS Number:20462-00-4
- Molecular formula:C7H12O2S2
- Molecular Weight:192.3

109-80-8
325 suppliers
$10.00/1g
6065-82-3
224 suppliers
$5.00/1g

20462-00-4
118 suppliers
$30.00/1g
Yield: 94%
Reaction Conditions:
with boron trifluoride diethyl etherate in chloroform for 1.5 h;Heating / reflux;
Steps:
B.1
Step 1 - To a solution of BF3. Et2O (57.5 mL, 0.454 mmol) in CHC13 (180 mL) heated at reflux was added dropwise, over a 1-h period, a solution of 1,3-propanedithiol (22.7 mL, 0.227 mmol), followed by ethyl diethoxyacetate (40 g, 0.227 mmol) in CHCl3 (40 mL). The resulting mixture was heated for 30 minutes, and then cooled to rt. The cooled solution was washed 2 times with water, once with saturated aqueous NaHC03, and then re-washed with water. The combined organic phases were dried over MgS04, then evaporated to give 41 g (94%) of ethyl 1,3-dithiane- 2-carboxylate as a yellow oil, which was used in the next step without purification. ¹H NMR (CDCl3) : No. 4.24 (2H, q, J = 7.2 Hz), 4.17 (1H, s), 3.46-3.39 (2H, m), 2.64-2.58 (2H, m), 2.18-2.01 (2H, m), 1.30 (3H, t, J = 7.2 Hz).
References:
BAYER PHARMACEUTICALS CORPORATION WO2005/99705, 2005, A2 Location in patent:Page/Page column 48

109-80-8
325 suppliers
$10.00/1g
515-84-4
242 suppliers
$36.00/100g

20462-00-4
118 suppliers
$30.00/1g