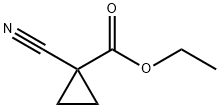
ETHYL 1-CYANOCYCLOPROPANECARBOXYLATE synthesis
- Product Name:ETHYL 1-CYANOCYCLOPROPANECARBOXYLATE
- CAS Number:1558-81-2
- Molecular formula:C7H9NO2
- Molecular Weight:139.15

106-93-4

105-56-6

1558-81-2
The general procedure for the synthesis of ethyl 1-cyanocyclopropanecarboxylate from 1,2-dibromoethane and ethyl cyanoacetate was as follows: a suspension of ethyl cyanoacetate (11.3 g, 0.1 mol), 1,2-dibromoethane, tetrabutylammonium bromide (Na 4 (n-Bu)4Br) and potassium carbonate (K 2 CO 3 ) in N,N-dimethylformamide (DMF, 100 mL) was heated to 80 °C and reacted overnight. Upon completion of the reaction, the mixture was poured into water (600 mL) and extracted with ethyl acetate (EtOAc, 3 x 50 mL). The organic layers were combined, washed with saturated saline, dried over anhydrous sodium sulfate (Na 2 SO 4 ) and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 10:1) to afford ethyl 1-cyanocyclopropanecarboxylate (10 g, yield: 72%).1H NMR (CDCl3, 400 MHz): δ 4.21 (q, J = 7.2 Hz, 2H), 1.65-1.61 (m, 2H), 1.58-1.55 (m, 2H) , 1.28 (t, J = 7.2 Hz, 3H).
Yield:1558-81-2 59.6%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80;
Steps:
CXI
To a stirred mixture of compound CXI-5 (30 g, 0.265 mol), l-bromo-2- chloroethane (75 g, 0.528 mol) in DMF (200 mL) was added K2C03 (110 g, 0.8 mol). The reaction mixture was heated to 80°C overnight. The mixture was diluted with ice water, extracted with hexane, washed with water and brine, dried over Na2S04, filtered and concentrated. The residue was main compound CXI-6 (22 g, yield 59.6%) and used directly.
References:
WO2013/25733,2013,A1 Location in patent:Paragraph 0793

107-06-2
440 suppliers
$14.00/25g

105-56-6
461 suppliers
$10.00/5ml

1558-81-2
161 suppliers
$8.00/1g
![Propanedinitrile, 2-[2-(methylthio)ethyl]-](/CAS/20210305/GIF/20144-11-0.gif)
20144-11-0
0 suppliers
inquiry

1558-81-2
161 suppliers
$8.00/1g

![Sulfilimine, S-ethenyl-S-(4-methylphenyl)-N-[(4-methylphenyl)sulfonyl]-, (+)- (9CI)](/CAS/20210305/GIF/81115-90-4.gif)