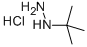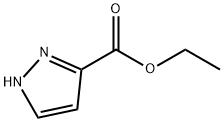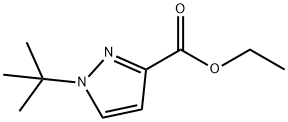
ethyl 1-tert-butyl-1H-pyrazole-3-carboxylate synthesis
- Product Name:ethyl 1-tert-butyl-1H-pyrazole-3-carboxylate
- CAS Number:682757-49-9
- Molecular formula:C10H16N2O2
- Molecular Weight:196.25
Yield:682757-49-9 76%
Reaction Conditions:
with triethylamine in ethanol at 25; for 12 h;
Steps:
65
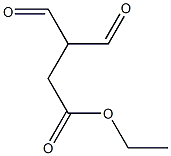
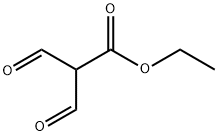
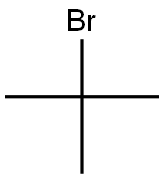
Method 65; Ethyl 1 -fert-butyl- lH-pyrazole-3 -carboxylate; A solution of ethyl 3-formyl-4-oxobutanoate (Method 64; 350 mg, 2.2 mmol) in EtOH (5 ml) was treated with triethylamine (465 μL, 3.3 mmol) and t-butyl hydrazine hydrochloride. The reaction stirred for 12 h at 25 °C. EtOH was removed under reduced pressure and the residue was redissolved in EtOAc and washed with H2O. The organics were dried with Na2SO4(S) and concentrated under reduced pressure. The residue was purified by column chromatography utilizing an Isco system (5% MeOH in CH2Cl2) to yield 327 mg (76 %) of an oil. H NMR (300 MHz): 1.29 - 1.35 (m, 3H), 1.57 (s, 9H), 4.25 (q, 2H), 7.86 (s, IH), 8.20 (s, IH).
References:
WO2007/71963,2007,A2 Location in patent:Page/Page column 58