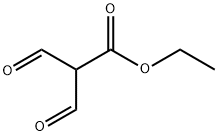
Propanoicacid,2-formyl-3-oxo-,ethylester synthesis
- Product Name:Propanoicacid,2-formyl-3-oxo-,ethylester
- CAS Number:80370-42-9
- Molecular formula:C6H8O4
- Molecular Weight:144.13
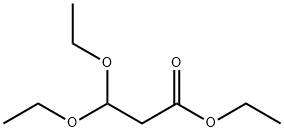
10601-80-6
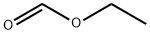
109-94-4

80370-42-9
Sodium hydride (2.46 g, 61.5 mmol) was accurately weighed in a dry 100-mL pear-shaped flask. It was washed sequentially with hexane and ether. The washed sodium hydride was suspended in ether (100 mL) and cooled in an ice bath to 0°C. Ethyl formate (24.84 mL, 308 mmol) was added slowly and dropwise, followed by ethyl 3,3-diethoxypropionate (5.98 mL, 30.8 mmol). The reaction mixture was stirred at room temperature for 15 hours to ensure complete reaction. After completion of the reaction, the mixture was slowly poured into ice water and the aqueous phase was washed with ether. The pH of the aqueous phase was adjusted to 1 with dilute hydrochloric acid and subsequently extracted with dichloromethane. The organic phases were combined, washed with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to afford ethyl 2-formyl-3-oxopropanoate (3.29 g, 22.83 mmol, 74.2% yield) as a golden-yellow slurry.

10601-80-6
315 suppliers
$6.00/5g
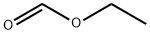
109-94-4
506 suppliers
$10.00/10g

80370-42-9
230 suppliers
$6.00/1g
Yield:80370-42-9 100%
Reaction Conditions:
with sodium hydride in diethyl ether at 0 - 20; for 15 h;
Steps:
28 Production of (ethoxycarbonyl)malondialdehyde
12.6 g of sodium hydride (purity: 60%, 525.0 mmoles) was washed with diethyl ether by decantation several times and then made into a solution in 500 ml of diethyl ether. Thereto were added, in a nitrogen current at 0 to 10°C, 194 g (2.6 moles) of ethyl formate and 50 g (262.0 mmoles) of ethyl 3,3-diethoxy-propionate. The resulting mixture was stirred at room temperature for 15 hours to give rise to a reaction. After confirmation of the completion of the reaction, the reaction mixture was poured into water, followed by washing with diethyl ether. The resulting aqueous layer was allowed to have a pH of 1 with hydrochloric acid, followed by extraction with dichloromethane. The resulting organic layer was washed with an aqueous sodium chloride solution and then dried over anhydrous magnesium sulfate. The resulting solution was subjected to vacuum distillation to remove the solvent contained therein, to obtain 37.6 g (yield: 100%) of crude (ethoxycarbonyl)malondialdehyde as a dark red oily substance. 1H-NMR [CDCl3/TMS, δ (ppm)]: 9.09 (2H,s), 5.26 (1H,s), 4.27 (2H,q), 1.28 (3H,t)
References:
KUMIAI CHEMICAL INDUSTRY CO., LTD.;IHARA CHEMICAL INDUSTRY CO., LTD. EP1364946, 2003, A1 Location in patent:Page/Page column 196
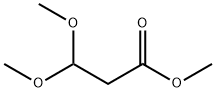
7424-91-1
263 suppliers
$9.00/1g

80370-42-9
230 suppliers
$6.00/1g

7424-91-1
263 suppliers
$9.00/1g
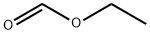
109-94-4
506 suppliers
$10.00/10g

80370-42-9
230 suppliers
$6.00/1g

10601-80-6
315 suppliers
$6.00/5g

80370-42-9
230 suppliers
$6.00/1g