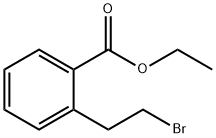
ethyl 2-(2-broMoethyl)benzoate synthesis
- Product Name:ethyl 2-(2-broMoethyl)benzoate
- CAS Number:179994-91-3
- Molecular formula:C11H13BrO2
- Molecular Weight:257.12
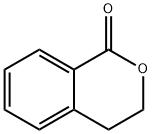
4702-34-5
101 suppliers
$10.00/250mg

64-17-5
825 suppliers
$10.00/50g

179994-91-3
17 suppliers
$517.76/5g
Yield:179994-91-3 78%
Reaction Conditions:
Stage #1: isochroman-1-onewith bromine in tetrachloromethane at 20 - 60; for 19 h;
Stage #2: ethanol in tetrachloromethane at 0; for 1 h;
Steps:
74.2 Step 2: ethyl 2-(2-bromoethyl)benzoate
Phosphorus tribromide (1.05 mL, 11.0 mmol) and bromine (0.62 mL, 12 mmol) were slowly added to a solution of 3,4-dihydro-1H-2-benzopyran-1-one (1.5 g, 10. mmol) in carbon tetrachloride (15 mL) cooled in an ice-water bath. The mixture was then stirred at room temperature for 16 hours. The resulting orange suspension was stirred at 60° C. for 3 hours. The red mixture was then cooled down and ethanol (10 mL) was slowly added at 0° C. (exothermic). The reaction mixture (orange solution) was stirred for one hour. The reaction mixture was partitioned between dichloromethane (50 mL) and water (20 mL). The organic phase was separated and washed with brine (10 mL), dried over sodium sulfate and concentrated under reduced pressure. The residue was purified by silica chromatography, eluting with a mixture of ethyl acetate and heptanes (0 to 10%) to afford ethyl 2-(2-bromoethyl)benzoate (2.0 g, 78% yield) as a pale yellow oil. 1H NMR (300 MHz, CDCl3) ppm 1.41 (t, J=6.9 Hz, 3H), 3.50 (t, J=6.9 Hz, 2H), 3.64 (t, J=6.9 Hz, 2H), 4.38 (q, J 6.9 Hz, 2H), 7.27-7.39 (m, 2H), 7.45 (t, J=7.5 Hz, 1H), 7.96 (d, J=7.5 Hz, 1H).
References:
US2016/95858,2016,A1 Location in patent:Paragraph 4485

4702-34-5
101 suppliers
$10.00/250mg

179994-91-3
17 suppliers
$517.76/5g
493-05-0
216 suppliers
$6.00/5g

179994-91-3
17 suppliers
$517.76/5g