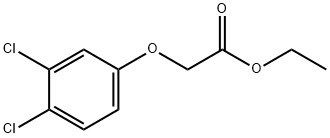
ethyl 2-(3,4-dichlorophenoxy)acetate synthesis
- Product Name:ethyl 2-(3,4-dichlorophenoxy)acetate
- CAS Number:62855-72-5
- Molecular formula:C10H10Cl2O3
- Molecular Weight:249.09
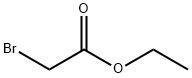
95-77-2
250 suppliers
$25.00/25g
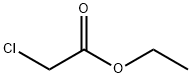
105-39-5
426 suppliers
$10.00/5g

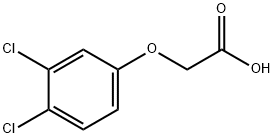
62855-72-5
15 suppliers
$437.00/1g
Yield:62855-72-5 100%
Reaction Conditions:
with potassium carbonate in acetone at 80; for 18 h;
Steps:
3 Step-1 : Synthesis of ethyl 2-(3,4-dichlorophenoxy)acetate
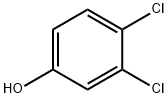
To a solution of 3,4-dichloro phenol (15.0 g, 0.0920 mol) in Acetone (150 mL) was added K2CO3(17.8 g, 0.128 mol) at room temperature. Ethyl chloro acetate (10.6 mL, 0.101 mol) was drop wise added to the reaction mixture at room temperature. The reaction mixture was heated at 80 °C for 18 h. Reaction was monitored by TLC (10% ethyl acetate in hexane). The resulting reaction mixture was allowed to cool to room temperature, poured into water (300 mL) and extracted with Ethyl acetate (4 x 100 mL). The combined organic phase was washed with brine solution (50 mL), dried over sodium sulfate, filtered and concentrated under vacuum yielding ethyl 2-(3,4-dichlorophenoxy)acetate (23 g, 0.0927 mol, 100%). This material was directly used for next step without any further purification.[2023] NMR (400 MHz, DMSO-d6) d ppm: 7.52 (d, J = 9.2 Hz, 1H), 7.26 (d, J = 2.8 Hz, 1H), 6.97 (dd, J = 8.8, 2.8 Hz, 1H), 4.85 (s, 2H), 4.19 - 4.13 (m, 2H), 1.20 (t, J = 8.4 Hz, 3H).
References:
WO2020/172324,2020,A1 Location in patent:Paragraph 0646; 2020-2023
588-22-7
70 suppliers
inquiry

62855-72-5
15 suppliers
$437.00/1g