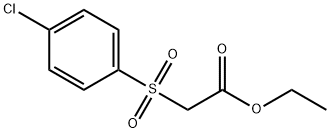
ETHYL 2-[(4-CHLOROPHENYL)SULPHONYL]ACETATE synthesis
- Product Name:ETHYL 2-[(4-CHLOROPHENYL)SULPHONYL]ACETATE
- CAS Number:3636-65-5
- Molecular formula:C10H11ClO4S
- Molecular Weight:262.71
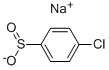
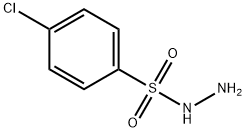
14752-66-0
160 suppliers
$7.00/1g

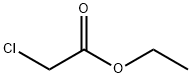
141-97-9
825 suppliers
$5.00/25g
![ETHYL 2-[(4-CHLOROPHENYL)SULPHONYL]ACETATE](/CAS/GIF/3636-65-5.gif)
3636-65-5
18 suppliers
$279.23/1gm:
Yield: 85%
Reaction Conditions:
with ammonium iodide in dimethyl sulfoxide at 20; for 7 h;Electrolysis;
Steps:
4.17 A typical procedure for the electrosynthesis of β-keto sulfones
General procedure: In a typical experiment, 1,3-dicarbonyl compounds (1 mmol), sodium sulfinates (1.2 mmol), DMSO (8 mL) and NH4I (4 mmol) were added to the undivided cell. The electrosynthesis was carried out in the undivided cell fitted with a Ni sheet cathode (2 cmx2.5 cmx0.02 cm) and a graphite rod anode at a constantcurrent (50 mA) at room temperature under magnetic stirring for 2 h and then continuously stirring for 5 h. After the reaction was finished, the electrolyte solution was decolorized with Na2S2O3, and then washed with distilled water (50 mL) and extracted withethyl acetate (10 mLx3). The solvent was removed under reduced pressure, and the crude product was purified by column chromatography on silica gel using petroleum ether-ethyl acetate (5:1) as eluent.
References:
Pan, Xiao-Jun;Gao, Jian;Yuan, Gao-Qing [Tetrahedron,2015,vol. 71,# 34,p. 5525 - 5530]
![ETHYL 2-[(4-CHLOROPHENYL)THIO]ACETATE](/CAS/GIF/52377-68-1.gif)
52377-68-1
26 suppliers
$75.00/100mg
![ETHYL 2-[(4-CHLOROPHENYL)SULPHONYL]ACETATE](/CAS/GIF/3636-65-5.gif)
3636-65-5
18 suppliers
$279.23/1gm:
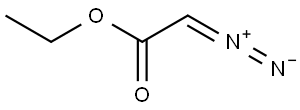
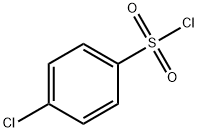
623-73-4
151 suppliers
$26.69/5g

14752-66-0
160 suppliers
$7.00/1g
![ETHYL 2-[(4-CHLOROPHENYL)SULPHONYL]ACETATE](/CAS/GIF/3636-65-5.gif)
3636-65-5
18 suppliers
$279.23/1gm: