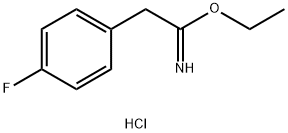
ethyl 2-(4-fluorophenyl)acetimidate hydrochloride synthesis
- Product Name:ethyl 2-(4-fluorophenyl)acetimidate hydrochloride
- CAS Number:51627-98-6
- Molecular formula:C10H13ClFNO
- Molecular Weight:217.67
Yield:51627-98-6 100%
Reaction Conditions:
with hydrogenchloride at 0 - 20; for 12 h;Inert atmosphere;
Steps:
B1
2-(4-Fluorophenyl)acetonitrile (1.0 g, 7.4 mmol) was dissolved in anhydrous ethanol (2 mL) under an atmosphere of Ar. The solution was cooled to 0 °C, saturated with HC1 gas, stirred for 6 h, allowed to warm to RT, and stirred for 6 h. Organic solvent was removed in vacuo to afford ethyl 2-(4-fluorophenyl)acetimidate hydrochloride (1.6 g, 100 %) as a white solid. H NMR (400 MHz, DMSO-i: δ 12.27 (m, 1 H), 11.20 (m, 1 H), 7.40 (dd, J= 8.3, 5.5 Hz, 2 H), 7.19 (t, J = 8.8 Hz, 2 H), 4.38 (q, J= 7.0 Hz, 2 H), 3.99 (s, 2 H), 1.25 (t, J= 7.0 Hz, 3 H).
References:
WO2014/145023,2014,A1 Location in patent:Paragraph 0382
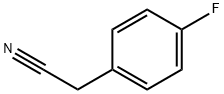
459-22-3
329 suppliers
$6.00/5g

51627-98-6
6 suppliers
inquiry
