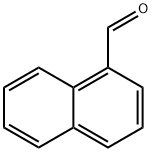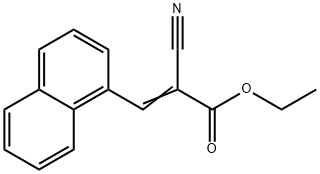
Ethyl 2-Cyano-3-(1-naphthalenyl)acrylate synthesis
- Product Name:Ethyl 2-Cyano-3-(1-naphthalenyl)acrylate
- CAS Number:7498-85-3
- Molecular formula:C16H13NO2
- Molecular Weight:251.28
Yield:7498-85-3 97.4%
Reaction Conditions:
with Fe3O4(at)UiO-66-NH2 core-shell nanohybrid (0.21 mmol -NH2, 3%) in N,N-dimethyl-formamide at 80; for 4 h;Knoevenagel Condensation;Time;
Steps:
2.3. Knoevenagel condensation
General procedure: Knoevenagel reactions were performed in a 25-mL round-bottomed flask equipped with a magnetic stirrer. In a typical experiment, a desired amount of catalyst (0 or 3% -NH2) and benzaldehyde (8 mmol) were mixed in DMF (5 mL). The flask was placed in an oil bath that had been preheated to 80 °C. The reaction was started by adding ethyl cyanoacetate (7 mmol) to the flask. The reaction was monitored using a gas chromatograph (GC-2010 plus, Shimadzu) equipped with an Rxi(at)-1 capillary column (30 m × 0.25 mm × 0.5 μm) and a flame ionization detector. The temperature program for gas chromatographic analysis was set as follows: the temperature was held at 150 °C for 5 min, then raised to 180 °C at 30 °C/min and held for 15 min. The inlet and detector temperatures were 280 °C. The analysis was performed directly after sampling to avoid any additional conversion. In the recycling experiments, the catalyst was recovered magnetically when the reaction was finished, washed with DMF (3 × 5 mL), and reused directly without further purification for the next run with fresh benzaldehyde and ethyl cyanoacetate. The catalyst was used for four consecutive runs.
References:
Zhang, Yanmei;Dai, Tianlin;Zhang, Fan;Zhang, Jing;Chu, Gang;Quan, Chunshan [Cuihua Xuebao/Chinese Journal of Catalysis,2016,vol. 37,# 12,p. 2106 - 2113]
4780-79-4
244 suppliers
$6.00/5g

7498-85-3
21 suppliers
inquiry