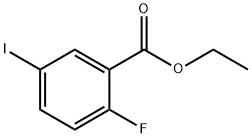
Ethyl-2-fluoro-5-iodobenzoate synthesis
- Product Name:Ethyl-2-fluoro-5-iodobenzoate
- CAS Number:773136-66-6
- Molecular formula:C9H8FIO2
- Molecular Weight:294.06

64-17-5
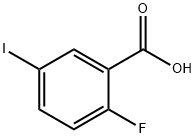
124700-41-0

773136-66-6
General procedure for the synthesis of ethyl 2-fluoro-5-iodobenzoate from ethanol and 2-fluoro-5-iodobenzoic acid: 2-Fluoro-5-iodobenzoic acid (Oakwood, 0.99 g, 3.72 mmol) and H2SO4 (198 μL, 3.72 mmol) were added to EtOH (7.4 mL). The reaction mixture was stirred at 100 °C overnight. After completion of the reaction, the mixture was concentrated in vacuum. The crude product was purified by fast column chromatography (gradient of 0 to 10% EtOAc/hexane) to afford the title compound ethyl 2-fluoro-5-iodobenzoate (916 mg, 84%) as a colorless oil. 1H NMR (CDCl3) δ 8.24 (dd, J = 6.8, 2.4 Hz, 1H), 7.81 (ddd, J = 8.8, 4.5, 2.4 Hz, 1H), 6.93 (dd, J = 10.3, 8.6 Hz, 1H), 4.41 (q, J = 7.0 Hz, 2H), 1.42 (t, J = 7.2 Hz, 3H).

64-17-5
825 suppliers
$10.00/50g

124700-41-0
244 suppliers
$11.00/1g

773136-66-6
47 suppliers
$22.00/250mg
Yield:773136-66-6 94.9%
Reaction Conditions:
with sulfuric acid at 100; for 5 h;
Steps:
1.1 (1) Compound 2 synthesis method:
Compound 1 (20.0 g, 75.2 mmol, 1 eq) was dissolved in 70 mL of EtOH.Then gradually add H2SO4 (7.37g, 75.1mmol, 4.01mL, 1eq).The mixture was stirred at 100 ° C for 5 h.And use TLC (Dichloromethane/Methanol=10/1, Rf=0.87) The progress of the reaction was detected.When the reaction is complete,The reactants were concentrated under reduced pressure.The concentrated product was extracted (EOtOH 200 mL, NaHCO 3 saturated solution (100.0 mL x 2), and then the organic layer was taken.It was dried by adding anhydrous Na2SO4.After further evaporation to dryness the title product 2 (21.0 g, 71.4 mmol, 94.9% yield).Figure 3 shows the results of TLC chromatography for this reaction.
References:
CN109942582,2019,A Location in patent:Paragraph 0057-0061