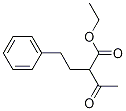
Ethyl 2-phenethylacetoacetate synthesis
- Product Name:Ethyl 2-phenethylacetoacetate
- CAS Number:5337-63-3
- Molecular formula:C14H18O3
- Molecular Weight:234.29

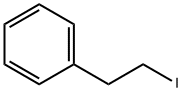
141-97-9
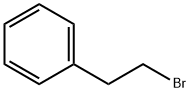
103-63-9

5337-63-3
The general procedure for synthesizing ethyl 2-phenylethylacetoacetate from ethyl acetoacetate and β-bromophenylethane is as follows: with reference to Example 3, the operation is the same as that of Reference Example 1, except that 55.5 g (300 mmol) of β-bromophenylethane, 42.9 g (330 mmol) of ethyl acetoacetate, 27.8 g of toluene, 103 g (748 mmol) of potassium carbonate, and 2.08 g of water as reactants. Upon completion of the reaction, 77.4 g of ethyl 2-phenylethyl acetoacetate crude product was obtained. The crude product was analyzed to contain 68.9 wt% of ethyl 2-phenylethylacetoacetate in 77% yield.
Yield:5337-63-3 88%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 60;
Steps:
18 Synthesis of 3-phenethyl-2-oxobutyric acid ethyl ester (10b)
60% sodium hydride (300 mg, 7.5 mmol, 1.0 equiv) was added to dry tetrahydrofuran (15 mL), ethyl acetoacetate (0.94 mL, 7.4 mmol, 1.0 equiv) and 10a (7.4 mmol, 1.0 equiv) were added dropwise, and the temperature was increased After the temperature reached 60°C and monitored by TLC, the solid in the reaction solution was filtered out, most of the solvent was evaporated, water (30 mL) was added, and ethyl acetate was added for three times. The organic phase was washed with saturated brine and dried over anhydrous sodium sulfate. , Filter, and evaporate the filtrate to obtain compound 10b with a yield of 88% as a yellow oil.
References:
CN113493449,2021,A Location in patent:Paragraph 0090-0092

103-63-9
504 suppliers
$9.00/10g

5337-63-3
21 suppliers
inquiry
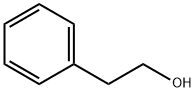
60-12-8
593 suppliers
$6.00/25g

5337-63-3
21 suppliers
inquiry