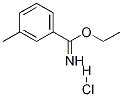
ethyl 3-methylbenzenecarboximidate hydrochloride synthesis
- Product Name:ethyl 3-methylbenzenecarboximidate hydrochloride
- CAS Number:54998-35-5
- Molecular formula:C10H14ClNO
- Molecular Weight:200
Yield:54998-35-5 93%
Reaction Conditions:
with acetyl chloride at 0 - 20; for 24 h;
Steps:
1 General procedure I for the synthesis of imidate hydrochlorides
General procedure: The benzonitrile 3 (15 mmol) was dissolved in ethanol(180 mmol) and cooled to 0 C. Acetyl chloride (120 mmol) wasadded drop wise to the reaction mixture. The reaction mixturewas stirred at room temperature for 24e48 h. Then, the reactionmixture was evaporated in vacuo. The crude product was washedwith diethyl ether (320 mL) and petroleum ether (320 mL).Recrystallization from ethanol afforded the imidate hydrochloride4.4.2.1
Ethyl-3-methylbenzimidate hydrochloride (4b)
Compound 3b (1.76 g, 15 mmol) was reacted under the conditions of general procedure I for 24 h to deliver ethyl-3-methylbenzimidate hydrochloride (4b) as a white solid in 93% yield (2.80 g, 14.0 mmol): mp 131-133 °C; Rf 0.63 (EtOAc/CH3OH=4:1); IR (ATR) 2997, 2777, 1633, 1591, 1461, 1436, 1381, 1356, 1121, 1072, 875, 857, 729, 670 cm-1; UV (CH3CN) λmax (log ε) 248 nm (4.02); 1H NMR (300 MHz, CD3OD) δ 1.62 (t, 3J=6.9 Hz, 3H, 4'-H), 2.47 (s, 1"-H), 4.67 (q, 3J=6.9 Hz, 2H, 3'-H), 7.53 (t-like, 3J (4-H, 5-H), (5-H, 6-H)=7.8 Hz, 1H, 5-H), 7.65 (d, 3J (4-H, 5-H)=7.8 Hz, 1H, 4-H), 7.88 (overlapped, 2H, 2-H and 6-H); 13C NMR (75 MHz, CD3OD) δ 13.9 (C-4'), 21.2 (C-1"), 71.3 (C-3'), 127.1 (C-1), 127.2 (C-2), 130.3 (C-6), 130.4 (C-5), 137.6 (C-4), 141.0 (C-3), 174.5 (C-1'); MS (ESI) m/z (%) 186 (10) [M-HCl+Na]+, 164 (55) [M-HCl+1]+, 136 (100), 119 (8); HRMS (ESI) calcd for C10H14NO 164.1075, found 164.1064.
References:
Sudheendran, Kavitha;Schmidt, Dietmar;Frey, Wolfgang;Conrad, Jürgen;Beifuss, Uwe [Tetrahedron,2014,vol. 70,# 8,p. 1635 - 1645]
620-22-4
226 suppliers
$16.00/25mL

54998-35-5
14 suppliers
inquiry

620-22-4
226 suppliers
$16.00/25mL

75-36-5
589 suppliers
$17.92/100G

54998-35-5
14 suppliers
inquiry
