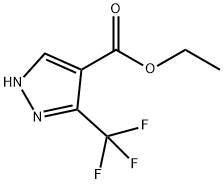
ETHYL 3-(TRIFLUOROMETHYL)PYRAZOLE-4-CARBOXYLATE synthesis
- Product Name:ETHYL 3-(TRIFLUOROMETHYL)PYRAZOLE-4-CARBOXYLATE
- CAS Number:155377-19-8
- Molecular formula:C7H7F3N2O2
- Molecular Weight:208.14
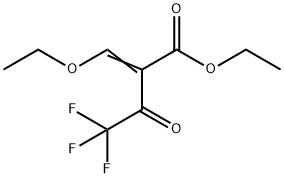
571-55-1

155377-19-8
The general procedure for the synthesis of ethyl 3-trifluoromethylpyrazole-4-carboxylate from ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutanoate was as follows: hydrazine hydrate (10 g, 0.199 mol) was dissolved in ethanol (400 mL) and the solution was cooled to 0 °C. A solution of ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutanoate (47.03 g, 0.19 mol) in ethanol (200 mL) was added dropwise to this cooled solution over 90 min. The reaction mixture was kept at 0 °C. Subsequently, the reaction solution was slowly warmed to room temperature and stirred continuously for 3 hours. Upon completion of the reaction, the mixture was concentrated to dryness. The resulting residue was dispersed in boiling cyclohexane (200 mL) and then cooled to room temperature. The precipitate was collected by filtration and washed with cyclohexane. Finally, the product was dried under vacuum at 75 °C to afford ethyl 3-trifluoromethylpyrazole-4-carboxylate (compound 3f) as a yellow powder (38.67 g, 95% yield). The melting point of the product was 145 °C. 1H NMR (CDCl3) data: δ 1.40 (t, 3H, J = 7.0 Hz), 4.39 (q, 2H, J = 7.0 Hz), 8.26 (s, 1H), 13.2 (s (br), 1H). 13C NMR (CDCl3) data: δ 14.0, 61.2, 113.4, 121.0 ( q, J = 268 Hz), 135.3, 142.0 (q, J = 38 Hz), 160.8. High-resolution mass spectrometry (HRMS) analysis: calculated value of 207.0381 for C7H7F3N2O2-H; measured value of 207.0377.

571-55-1
162 suppliers
$10.00/1g

155377-19-8
177 suppliers
$6.00/250mg
Yield:155377-19-8 95%
Reaction Conditions:
with hydrazine hydrate in ethanol at 0 - 20; for 4.5 h;
Steps:
2.10. Ethyl 3-(trifluoromethyl)-1H-pyrazole-4-carboxylate (3f)
Hydrazine hydrate (10 g, 0.199 mol) was dissolved in ethanol (400 mL). This was cooled to 0 °C and a solution of ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutanoate (47.03 g, 0.19 mol) in ethanol (200 mL) was added drop-wise over 90 min at 0 °C. The solution was left to warm to room temperature for 3 h and concentrated to dryness. The residue was dispersed in boiling cyclohexane (200 mL) and left to cool to room temperature. The precipitate was filtered, washed with cyclohexane and dried under vacuum at 75 °C to yield compound 3f as a yellow powder (38.67 g, 95%); mp 145 °C 1H NMR (CDCl3): 1.40 (t, 3H, J=7.0 Hz); 4.39 (q, 2H, J=7.0 Hz); 8.26 (s, 1H); 13.2 (s (br), 1H). 13C NMR (CDCl3): 14.0; 61.2; 113.4; 121.0 (q, J=268 Hz); 135.3; 142.0 (q, J=38 Hz); 160.8. HRMS: calcd For C7H7F3N2O2-H: 207.0381; Found: 207.0377.
References:
Guillou, Sandrine;Bonhomme, Frédéric J.;Ermolenko, Mikhail S.;Janin, Yves L. [Tetrahedron,2011,vol. 67,# 44,p. 8451 - 8457] Location in patent:experimental part
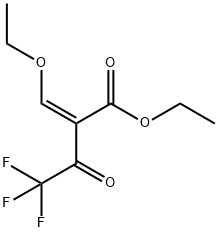
1048670-08-1
8 suppliers
inquiry

155377-19-8
177 suppliers
$6.00/250mg
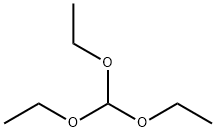
122-51-0
514 suppliers
$10.00/5ml
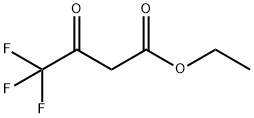
372-31-6
488 suppliers
$5.00/10g

155377-19-8
177 suppliers
$6.00/250mg

372-31-6
488 suppliers
$5.00/10g

155377-19-8
177 suppliers
$6.00/250mg
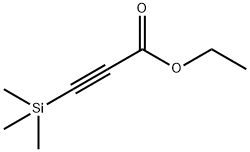
16205-84-8
82 suppliers
$41.00/5g

155377-19-8
177 suppliers
$6.00/250mg