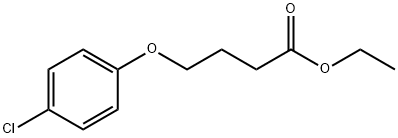
ethyl 4-(4-chlorophenoxy)butanoate synthesis
- Product Name:ethyl 4-(4-chlorophenoxy)butanoate
- CAS Number:59227-79-1
- Molecular formula:C12H15ClO3
- Molecular Weight:242.7
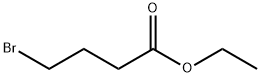
2969-81-5
458 suppliers
$5.00/1g
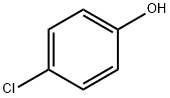
106-48-9
498 suppliers
$10.00/5G

59227-79-1
26 suppliers
$59.00/250mg
Yield:59227-79-1 92%
Reaction Conditions:
with potassium carbonate in acetonitrile; for 8 h;Reflux;
Steps:
51.1 Step 1 :
To a solution of 4-chlorophenol (2.0 g, 15.556 mmol, 1 equiv) in acetonitrile (60 mL) was added anhydrous potassium carbonate (4.3 g, 31 .1 13 mmol, 2 equiv) and ethyl 4-bromobutanoate (3.56 mL, 24.891 mmol, 1 .6 equiv). The reaction mixture was heated to reflux and stirred for 8 h. The progress of the reaction was monitored by TLC. After completion of reaction, the reaction mixture was allowed to cool to 27 °C, filtered the solid and washed with ethyl acetate (100 mL). The filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography using 10% ethyl acetate in hexane as eluent to obtain the title compound ethyl 4-(4-chlorophenoxy)butanoate (3.5 g, 92 % yield) as colourless liquid. LCMS (ES) m/z = 242.9 [M+H]+. NMR (400 MHz, CDCI3): δ ppm 1 .25 (t, J = 6.8 Hz, 3 H), 2.06 - 2.06 (m, 2 H), 2.49 (t, J = 6.8 Hz, 2 H), 3.97 (t, J = 6.0 Hz, 2 H), 4.1 1 - 4.17 (m, 2 H), 6.80 (d, J = 8.4 Hz, 2 H), 7.21 (d, J = 8.8 Hz, 2 H).
References:
WO2017/212425,2017,A1 Location in patent:Page/Page column 112

64-17-5
825 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry
13997-70-1
40 suppliers
inquiry

59227-79-1
26 suppliers
$59.00/250mg

106-48-9
498 suppliers
$10.00/5G

59227-79-1
26 suppliers
$59.00/250mg