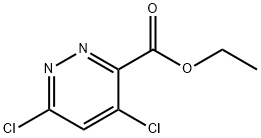
Ethyl 4,6-dichloropyrridazine-3-carboxylate synthesis
- Product Name:Ethyl 4,6-dichloropyrridazine-3-carboxylate
- CAS Number:679406-03-2
- Molecular formula:C7H6Cl2N2O2
- Molecular Weight:221.04
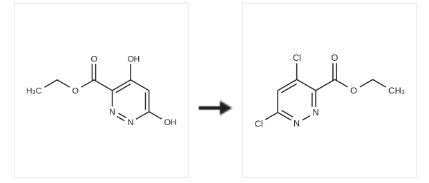
1352925-63-3
139 suppliers
$95.00/100mg

679406-03-2
144 suppliers
$32.00/100mg
Yield:679406-03-2 65%
Reaction Conditions:
with trichlorophosphate at 100; for 3 h;
Steps:
52.3
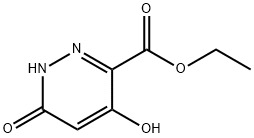
Step 3: Add 4,6-dihydroxypyridazine-3-carboxylate ethyl ester (52-c, 3.6 g, 19.5 mmol) to phosphorus oxychloride (50 ml), raise the temperature to 100 degrees, stir After 3 hours, concentrated under reduced pressure, the residue was poured into 50 ml of water, and extracted with ethyl acetate (20 ml of ethyl acetate, the organic layer was dried, filtered with suction, and concentrated. The residue was purified by column chromatography (ethyl acetate: petroleum ether=1: 4) 4,6-dichloropyridazine-3-carboxylic acid ethyl ester (52-d, 2.8 g, 12.7 mmol, 65% yield) was obtained without purification, and the next step was carried out directly.
References:
WO2022/117016,2022,A1 Location in patent:Page/Page column 104
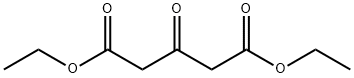
105-50-0
404 suppliers
$6.00/5g

679406-03-2
144 suppliers
$32.00/100mg