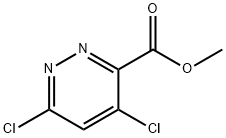
Methyl 4,6-dichloropyridazine-3-carboxylate synthesis
- Product Name:Methyl 4,6-dichloropyridazine-3-carboxylate
- CAS Number:372118-01-9
- Molecular formula:C6H4Cl2N2O2
- Molecular Weight:207.01
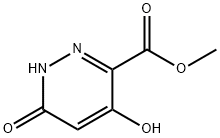
372118-00-8

372118-01-9
Step 3 Synthesis of methyl 4,6-dichloropyridazine-3-carboxylate: 4,6-dihydroxypyridazine-3-carboxylic acid methyl ester (10.5 g, 61.7 mmol) was mixed with phosphorous trichloride (70 mL), heated to 95 °C, and the reaction was carried out for 5 hours. After completion of the reaction, the excess phosphorous trichloride was evaporated under reduced pressure. The crude product was slowly poured into ice water (250 mL) and extracted with ethyl acetate (3 x 100 mL). The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography (100-200 mesh, 30% ethyl acetate in hexane solution as eluent) to afford methyl 4,6-dichloropyridazine-3-carboxylate (9.2 g, 72% yield) as an off-white solid.LC-MS: 207.0 [M + H]+.

372118-00-8
80 suppliers
$39.00/100mg

372118-01-9
201 suppliers
$11.00/250mg
Yield:372118-01-9 72%
Reaction Conditions:
with trichlorophosphate at 95; for 5 h;
Steps:
17.3 4,6-Dichloro-pyridazine-3-carboxylic acid methyl ester
Step 3
4,6-Dichloro-pyridazine-3-carboxylic acid methyl ester
A mixture of 4,6-dihydroxy-pyridazine-3-carboxylic acid methyl ester (10.5 g, 61.7 mmol) and POCl3 (70 mL) was heated to 95° C. for 5 h.
The excess POCl3 was removed under reduced pressure, then the crude residue was added to ice-water (250 mL) and extracted with ethyl acetate (3*100 mL).
The combined extracts were dried then concentrated to give a crude residue which was purified by chromatography (silica, 100-200 mesh, 30% ethyl acetate in hexane) to give 4,6-dichloro-pyridazine-3-carboxylic acid methyl ester (9.2 g, 72%) as an off white solid. LC-MS: 207.0 [M+H]+.
References:
Hoffman-La Roche Inc.;Hermann, Johannes Cornelius;Kennedy-Smith, Joshua;Lucas, Matthew C.;Padilla, Fernando;Soth, Michael US2013/178478, 2013, A1 Location in patent:Paragraph 0301;0302
1830-54-2
410 suppliers
$9.00/10g

372118-01-9
201 suppliers
$11.00/250mg
83878-89-1
3 suppliers
inquiry

372118-01-9
201 suppliers
$11.00/250mg