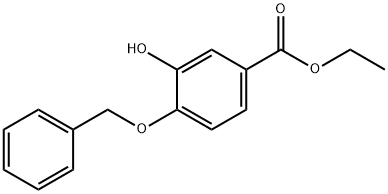
Ethyl 4-(benzyloxy)-3-hydroxybenzoate synthesis
- Product Name:Ethyl 4-(benzyloxy)-3-hydroxybenzoate
- CAS Number:177429-27-5
- Molecular formula:C16H16O4
- Molecular Weight:272.3
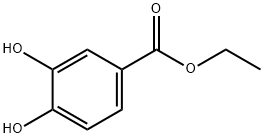
3943-89-3

100-44-7

177429-27-5
Step 9a: Synthesis of ethyl 4-(benzyloxy)-3-hydroxybenzoate (compound 502) A mixture of ethyl 3,4-dihydroxybenzoate (9.1 g, 50 mmol), benzyl chloride (6.3 g, 50 mmol), potassium iodide (1.66 g, 10 mmol) and potassium carbonate (13.8 g, 100 mmol) in acetonitrile (250 mL) was reacted by stirring at 40 °C overnight. Upon completion of the reaction, the mixture was cooled to room temperature and filtered. The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography with petroleum ether/ethyl acetate (10:1, v/v) as eluent to give the title compound 502 as a white solid (4.4 g, 33% yield). LCMS (m/z): 273 [M+1]+. 1H NMR (DMSO-d6): δ 9.49 (s, 1H), 7.35 (m, 7H), 7.08 (d, J = 7.8 Hz, 1H), 5.16 (s, 2H), 4.21 (m, 2H), 1.26 (t, J = 7.5 Hz, 3H).

1195945-43-7
0 suppliers
inquiry

177429-27-5
20 suppliers
$56.00/100mg
Yield:177429-27-5 96%
Reaction Conditions:
with water;sodium carbonate in tetrahydrofuran;methanol at 20;Inert atmosphere;
Steps:
3.3.3. Ethyl 3-hydroxy-4-benzyloxybenzoate (4d)
Compound 7 (2.00 g, 6.36 mmol) was dissolved in MeOH/THF (2:1, 45 mL). A solution of sodium carbonate (0.81 g. 7.64 mmol in 15 mL of H2O) was added. The mixture was stirred overnight at room temperature and then concentrated. The residue was partitioned between EtOAc and water. The organic phase was washed with brine and dried (MgSO4). Solvent removal under vacuum gave the product as a white solid (1.66 g, 96%); mp 82-84 °C; νmax (KBr disc, cm-1) 3383 (OH), 1686 (CO); δH (400 MHz, CDCl3) 7.63 (d, 1H, J 1.9 Hz, H-2); 7.61 (dd, 1H, J 1.9, 8.3 Hz, H-6), 7.45-7.35 (5H, m, Bn H), 6.95 (d, 1H, J 8.3 Hz, H-5), 5.70 (br s, 1H, OH), 5.18 (s, 2H, Bn CH2), 4.35 (q, 2H, J 7.1 Hz, CH2CH3), 1.40 (t, 3H, J 7.1 Hz, CH3); δC (100.6 MHz, CDCl3) 149.9, 145.4, 135.6, 128.9, 128.7, 127.9, 124.1, 122.6, 115.8, 111.2, 71.1, 60.8, 14.4; m/z (ESI+) 295 [M+Na]+, HRESI+ 295.0945, C16H16NaO4 requires 295.0946.
References:
Zhang, Qingzhi;Raheem, K. Saki;Botting, Nigel P.;Slawin, Alexandra M.Z.;Kay, Colin D.;O'Hagan, David [Tetrahedron,2012,vol. 68,# 22,p. 4194 - 4201] Location in patent:experimental part

3943-89-3
441 suppliers
$6.00/5g

100-39-0
438 suppliers
$10.00/10g

177429-27-5
20 suppliers
$56.00/100mg

3943-89-3
441 suppliers
$6.00/5g

100-51-6
1446 suppliers
$5.00/100g

177429-27-5
20 suppliers
$56.00/100mg

3943-89-3
441 suppliers
$6.00/5g

100-44-7
658 suppliers
$13.50/250G

177429-27-5
20 suppliers
$56.00/100mg

3943-89-3
441 suppliers
$6.00/5g

100-39-0
438 suppliers
$10.00/10g
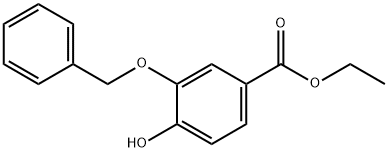
188564-63-8
3 suppliers
inquiry

177429-27-5
20 suppliers
$56.00/100mg

174398-83-5
21 suppliers
inquiry