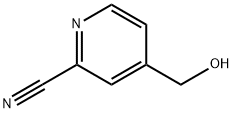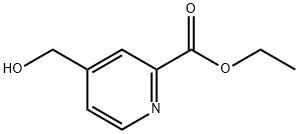
ethyl 4-(hydroxyMethyl)picolinate synthesis
- Product Name:ethyl 4-(hydroxyMethyl)picolinate
- CAS Number:59663-96-6
- Molecular formula:C9H11NO3
- Molecular Weight:181.19
Yield:59663-96-6 16%
Reaction Conditions:
Stage #1: ethanol;2-Cyano-4-(hydroxymethyl)pyridinewith chloro-trimethyl-silane at 50; for 12 h;
Stage #2: with water;sodium carbonate
Steps:
12 2-Ethoxycarbonylpyridine-4-methanol (Reference compound No.12-1)
Reference Example 12 2-Ethoxycarbonylpyridine-4-methanol (Reference compound No.12-1) Trimethylsilyl chloride (0.4 mL, 3.0 mmol) was added to a solution of 2-cyanopyridine-4-methanol (200 mg, 1.5 mmol, Reference compound No.11-1) in ethanol (3 mL) at 50°C under a nitrogen atmosphere, then the mixture was stirred for 12 hours. The mixture was allowed to stand, and a little water and sodium carbonate (160 mg, 1.5 mmol) were added thereto. The solution was dried over anhydrous magnesium sulfate, then the solvent was evaporated under reduced pressure. The resulting residue was purified by silica gel column chromatography to give 43 mg of the title reference compound as a white solid. (Yield 16%) 1H-NMR(500MHz,DMSO-d6) δ 1.33(t,J = 7.0 Hz,3H),4.35(q,J = 7.0 Hz,2H),4.62(d,J = 5.8 Hz,2H),5.56(t,J = 5.8 Hz,1H),7.55(m,1H),8.01(dd,J = 1.6,0.6 Hz,1H),8.63(dd,J = 4.6, 0.6 Hz,1H)
References:
EP1602647,2005,A1 Location in patent:Page/Page column 53
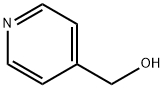
586-95-8
375 suppliers
$6.00/1g

59663-96-6
32 suppliers
inquiry
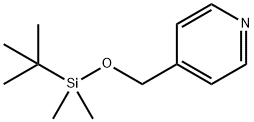
117423-41-3
42 suppliers
$75.00/100mg

59663-96-6
32 suppliers
inquiry

117423-42-4
0 suppliers
inquiry

59663-96-6
32 suppliers
inquiry