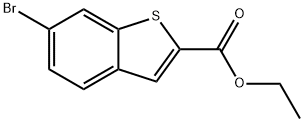
Ethyl 6-broMo-1-benzothiophene-2-carboxylate synthesis
- Product Name:Ethyl 6-broMo-1-benzothiophene-2-carboxylate
- CAS Number:105191-64-8
- Molecular formula:C11H9BrO2S
- Molecular Weight:285.16
57848-46-1
383 suppliers
$9.00/1g

105191-64-8
59 suppliers
$55.00/250mg
Yield: 86%
Reaction Conditions:
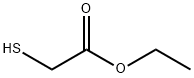
Stage #1:ethyl 2-sulfanylacetate with sodium hydride in dimethyl sulfoxide for 0.166667 h;
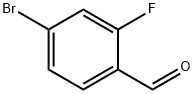
Stage #2:4-bromo-2-fluorobenzaldehyde in dimethyl sulfoxide for 0.25 h;
Steps:
35.1
Step 1 6-bromo-benzorb1thiophene-2-carboxylic acid ethyl esterSodium hydride (1.41 g, 35.32 mmol) is added to a round bottom flask and washed with hexanes (10 mL) twice. To the flask is added dimethyl sulfoxide (30 mL) and ethyl 2-mercaptoacetate (3.54 g, 29.43 mmol). The mixture is stirred for 10 minutes and 4-bromo-2-fluoro-benzaldehyde (4.78 g; 23.55 mmol) is added. The reaction mixture is stirred for 15 minutes and quenched with ice-water (100 g). The mixture is extracted with CHaCl2 (50 mL x2). The combined organic layers are dried over Na2SO^ filtered, and concentrated under reduced pressure. The crude product is purified via flash chromatography eluting with 10% EtOAc/Hexanes. The appropriate fractions are combined and concentrated under reduced pressure to afford the title compound (5.75 g, 86%). 1H NMR (400 MHz, CDCl3): δ 8.00 (s, 1 H), 7.99 (s, 1 H), 7.75 (d, IH), 7.48 (d, IH), 4.38 (q, 2H), 1.39 (t, 3H).
References:
ELI LILLY AND COMPANY WO2007/92751, 2007, A2 Location in patent:Page/Page column 29
623-51-8
247 suppliers
$7.00/25g

57848-46-1
383 suppliers
$9.00/1g

105191-64-8
59 suppliers
$55.00/250mg

64-17-5
825 suppliers
$10.00/50g
105212-27-9
9 suppliers
$353.45/1gm:

105191-64-8
59 suppliers
$55.00/250mg
5629-98-1
86 suppliers
$8.00/250mg

105191-64-8
59 suppliers
$55.00/250mg
![6-BROMO-BENZO[B]THIOPHENE-2-CARBOXYLIC ACID](/CAS/GIF/19075-58-2.gif)
19075-58-2
133 suppliers
$39.00/500mg

105191-64-8
59 suppliers
$55.00/250mg