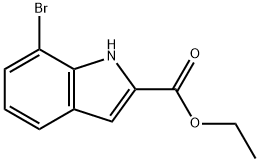
Ethyl 7-bromo-1H-indole-2-carboxylate synthesis
- Product Name:Ethyl 7-bromo-1H-indole-2-carboxylate
- CAS Number:16732-69-7
- Molecular formula:C11H10BrNO2
- Molecular Weight:268.11
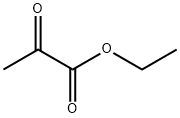
617-35-6
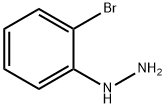
16732-66-4

16732-69-7
Example XIX- Synthesis of ethyl 7-bromo-1H-indole-2-carboxylate: 11.0 g of 2-bromophenylhydrazine and 550 mg of p-toluenesulfonic acid monohydrate were dissolved in 200 mL of toluene, 6.74 mL of ethyl pyruvate was added, and the reaction was carried out at reflux, also assembled with an aqueous separator, for 2 hours. Upon completion of the reaction, the mixture was cooled to 40°C and combined with a pre-prepared solution (prepared by dissolving 44.75 g of p-toluenesulfonic acid monohydrate in 300 mL of toluene, also fitted with a water separator and refluxed for 2 hours). The combined mixture was continued to reflux the reaction. At the end of the reaction, it was cooled to room temperature and the solvent was removed by distillation under reduced pressure. The residue was dissolved in ethyl acetate and washed sequentially with water and saturated aqueous sodium bicarbonate. The organic phase was dried with anhydrous magnesium sulfate, followed by the addition of activated carbon, stirring for 15 minutes and filtration through diatomaceous earth. This process was repeated twice to ensure purification. The filtrate was concentrated under reduced pressure and the residue was dissolved in a petroleum ether/dichloromethane (7:3) solvent mixture, 30 g of silica gel was added, stirred for 10 minutes and filtered through diatomaceous earth extraction. The silica gel was washed with petroleum ether/dichloromethane (7:3). Finally, the solvent was removed under reduced pressure to give 7.37 g of product in 47% yield. The product was analyzed by HPLC (Method 1): retention time = 3.82 min. mass spectrum (ESI+): m/z = 268 [M+H]+.

617-35-6
563 suppliers
$5.00/10g

16732-66-4
54 suppliers
inquiry

16732-69-7
124 suppliers
$9.00/100mg
Yield:16732-69-7 47%
Reaction Conditions:
toluene-4-sulfonic acid in toluene for 16 h;Reflux;Water separator;
Steps:
XIX Ethyl 7-bromo-1H-indole-2-carboxylate
EXAMPLE XIX Ethyl 7-bromo-1H-indole-2-carboxylate 11.0 g 2-bromophenylhydrazine and 550 mg p-toluenesulphonic acid monohydrate are dissolved in 200 ml of toluene, combined with 6.74 ml ethyl pyruvate and refluxed for 2 hours using the water separator. The mixture is allowed to cool to 40° C. and combined with a solution that is obtained by dissolving 44.75 g p-toluenesulphonic acid monohydrate in 300 ml of toluene and refluxing for two hours using the water separator. Then the mixture is refluxed for 12 hours using the water separator. After cooling to ambient temperature the solvents are eliminated in vacuo, the residue is taken up in ethyl acetate and washed successively with water and saturated aqueous sodium hydrogen carbonate solution. After drying with magnesium sulphate the mixture is combined with activated charcoal, stirred for 15 minutes and filtered through kieselguhr. The process of adding activated charcoal, stirring and filtering is repeated twice more. The solvents are eliminated in vacuo and the residue is taken up in petroleum ether/dichloromethane 7:3. 30 g of silica gel are added, the mixture is stirred for 10 minutes and then suction filtered through kieselguhr. The suction filtered silica gel is washed with petroleum ether/dichloromethane 7:3. The solvents are eliminated in vacuo. Yield: 7.37 g (47% of theory) HPLC (method 1): retention time=3.82 min. Mass spectrum (ESI+): m/z=268 [M+H]+
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH US2011/269737, 2011, A1 Location in patent:Page/Page column 49

617-35-6
563 suppliers
$5.00/10g
50709-33-6
293 suppliers
$5.00/1g

16732-69-7
124 suppliers
$9.00/100mg

50709-33-6
293 suppliers
$5.00/1g

16732-69-7
124 suppliers
$9.00/100mg
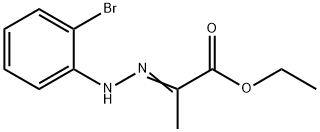
18474-55-0
22 suppliers
inquiry

16732-69-7
124 suppliers
$9.00/100mg