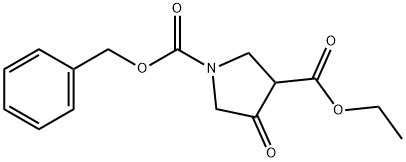
Ethyl N-Cbz-4-Oxopyrrolidine-3-carboxylate synthesis
- Product Name:Ethyl N-Cbz-4-Oxopyrrolidine-3-carboxylate
- CAS Number:51814-19-8
- Molecular formula:C15H17NO5
- Molecular Weight:291.3
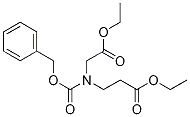
51814-17-6

51814-19-8
Ethyl 3-[N-benzyloxycarbonyl-N-(ethoxycarbonylmethyl)amino]propanoate (26.8 g, 79.5 mmol) was used as a raw material and dissolved in ethanol (200 mL). To this solution was added an ethanolic solution of 20% sodium ethanolate (40.6 mL, 119.3 mmol), followed by heating and refluxing the reaction mixture for 2 hours. Upon completion of the reaction, the solvent was removed by concentration under reduced pressure and the residue was dissolved in water (100 mL). The aqueous solution was acidified by slowly adding concentrated hydrochloric acid to the aqueous solution under cooling in an ice bath and extracted with chloroform (100 mL x 3) after acidification. The chloroform extracts were combined, washed with saturated aqueous sodium chloride solution (100 mL) and then dried with anhydrous sodium sulfate. After drying, the filtrate was filtered and the filtrate was concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate, 2:1) to give 16.7 g of ethyl N-Cbz-4-oxo-3-pyrrolidinecarboxylate in 72% yield as a light brown oil. The product was characterized by 1H-NMR (400 MHz, CDCl3) and MS (ESI): 1H-NMR δppm: 1.25-1.33 (3H, m), 3.87-4.37 (7H, m), 5.16-5.22 (2H, m), 7.23-7.41 (5H, m); MS (ESI) m/z: 314 (M + Na) + . .

51814-17-6
18 suppliers
$181.25/1g

51814-19-8
121 suppliers
$14.00/250mg
Yield:51814-19-8 72%
Reaction Conditions:
Stage #1:ethyl N-benzoxycarbonyl-N-(2-ethoxcarbonylethyl)glycinate with sodium ethanolate in ethanol for 2 h;Heating / reflux;
Stage #2: with hydrogenchloride;water at 0;
Steps:
63
Reference Example 63 Ethyl 1-benzyloxycarbonyl-4-oxopyrrolidine-3-carboxylate To a solution of ethyl 3-[N-benzyloxycarbonyl-N-(ethoxycarbonyl methyl)amino]ethylpropionate (26.8 g, 79.5 mmol) in ethanol (200 mL), sodium ethoxide (20% solution in ethanol, 40.6 mL, 119.3 mmol) was added, and the mixture was heated under reflux for 2 hours. After concentrating the reaction mixture under reduced pressure, the residue was dissolved in water (100 mL). Concentrated hydrochloric acid was added to this solution in an ice bath for acidification, and the solution was extracted with chloroform (100 mL*3). The extract was washed with saturated aqueous solution of sodium chloride (100 mL), and dried with anhydrous sodium sulfate. After the filtration, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (hexane:ethyl acetate, 2:1) to obtain 16.7 g (72%) of the title compound as a pale brown oily product. 1H-NMR (400 MHz, CDCl3)δ ppm: 1.25-1.33 (3H, m), 3.87-4.37 (7H, m), 5.16-5.22 (2H, m), 7.23-7.41 (5H, m). MS (ESI) m/z: 314 (M+Na)+.
References:
DAIICHI PHARMACEUTICAL CO., LTD. US2006/264428, 2006, A1 Location in patent:Page/Page column 47-48
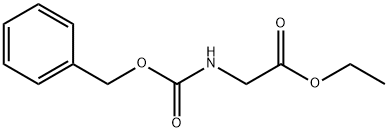
1145-81-9
156 suppliers
$139.00/5 g
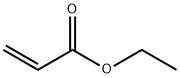
140-88-5
559 suppliers
$12.00/100ml

51814-19-8
121 suppliers
$14.00/250mg

140-88-5
559 suppliers
$12.00/100ml

51814-19-8
121 suppliers
$14.00/250mg

501-53-1
409 suppliers
$10.00/1g

51814-19-8
121 suppliers
$14.00/250mg