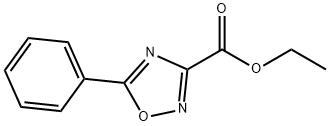
ETHYL5-PHENYL-1,2,4-OXADIAZOLE-3-CARBOXYLATE synthesis
- Product Name:ETHYL5-PHENYL-1,2,4-OXADIAZOLE-3-CARBOXYLATE
- CAS Number:37384-62-6
- Molecular formula:C11H10N2O3
- Molecular Weight:218.21
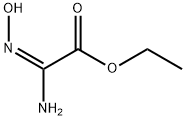
1217428-98-2
20 suppliers
inquiry
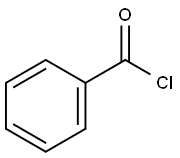
98-88-4
598 suppliers
$10.00/5g

37384-62-6
38 suppliers
$45.00/50mg
Yield:37384-62-6 80%
Reaction Conditions:
with pyridine in toluene;Reflux;
Steps:
NV930, already reported in the literature, has been prepared by a different synthetic procedure: NV930 has been prepared by the classic amidoxime route: The amidoxime 1 (0.3 g) (prepared as reported in (P. S. Branco, S. Prabhakar, A. M. Lobo, D. J. Williams, "Reactions of hydroxylamines with ethyl cyanoformate . preparation of aminonitrones and their synthetic applications . "tetrahedron 1992, 48, 6335-6360)) was dissolved in 50 mL of toluene in a 250 mL round bottomed flask. Then, 1.2 eq. of the appropriate aroyl chloride and 1.2 eq. of pyridine were added and the reaction mixture was refluxed for 6-8 h monitoring the reaction by TLC until consumption of starting material. The solvent was removed under vacuum and water was added to the residue. Extraction with ethyl acetate and chromatographic separation on silica gel using mixtures of petroleum ether and ethyl acetate as eluent allowed to obtain the desired oxadiazole, further purified by crystallization. (0116) Reaction Yield 80%, MP 50-52°C, from Ethanol. 1H NMR (300 MHz, CDC13 ) d : 8.22 (d, J = 7.2 Hz, 2H) , 7.64 (t, J = 7.4 Hz, 1H) , 7.55 (t, J = 7.4 Hz, 2H) , 4.55 (q, J = 7.1 Hz, 2H) , 1.47 (t, J = 7.1 Hz, 3H) . FT-IR cm-1, 1720. HRMS for C11H10N2O3 found 219.0699 [M+H]+ (Calcd. 219.0691) .
References:
WO2019/101709,2019,A1 Location in patent:Page/Page column 22-23
![Acetic acid, 2-[(benzoyloxy)amino]-2-imino-, ethyl ester](/CAS/20210111/GIF/1081533-58-5.gif)
1081533-58-5
0 suppliers
inquiry

37384-62-6
38 suppliers
$45.00/50mg

98-88-4
598 suppliers
$10.00/5g

37384-62-6
38 suppliers
$45.00/50mg

100-46-9
502 suppliers
$5.00/5G

37384-62-6
38 suppliers
$45.00/50mg
65-85-0
1436 suppliers
$10.00/25g

37384-62-6
38 suppliers
$45.00/50mg