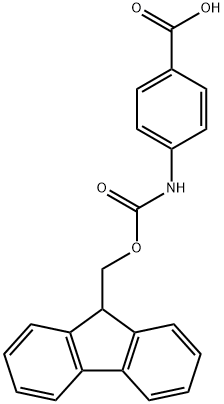
FMOC-4-AMINOBENZOIC ACID synthesis
- Product Name:FMOC-4-AMINOBENZOIC ACID
- CAS Number:185116-43-2
- Molecular formula:C22H17NO4
- Molecular Weight:359.37
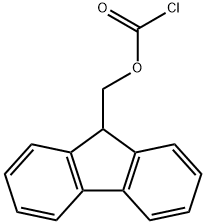
28920-43-6
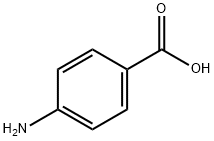
150-13-0

185116-43-2
Under an inert atmosphere, p-aminobenzoic acid (10 g, 73 mmol) was dissolved in anhydrous N-methylpyrrolidone (NMP, 50 mL). Subsequently, a solution of anhydrous NMP (50 mL) of 9-fluorenylmethyl chloroformate (Fmoc-Cl, 18.9 g, 73 mmol) was slowly added dropwise. The reaction mixture was stirred at room temperature for 24 hours. Upon completion of the reaction, the mixture was slowly poured into 400 mL of water to precipitate the product. The colorless precipitate was collected by filtration and washed with plenty of water. Finally, the product was dried under vacuum at 120 °C to afford N-Fmoc-p-aminobenzoic acid (24.8 g, 94% yield). The melting point of the product was 215 °C (decomposition). Nuclear magnetic resonance hydrogen spectrum (1H-NMR, 300 MHz, DMSO-d6): δ 4.33 (t, J = 6.25 Hz, 1H), 4.54 (d, J = 6.25 Hz, 2H), 7.33-7.45 (m, 4H), 7.57 (d, J = 7.35 Hz, 2H), 7.76 (d, J = 7.35 Hz, 2H), and 7.89 (m, 4H), 10.08 (s, 1H), 12.69 (s, 1H). NMR carbon spectrum (13C-NMR and DEPT, 300 MHz, DMSO-d6): δ 46.61 (CH2), 65.83 (CH), 117.5 (CH), 120.22 (CH), 124.48 (C), 125.14 (CH), 127.17 (CH), 127.74 (CH), 130.48 ( CH), 140.86 (C), 143.31 (C), 143.74 (C), 153.31 (C), 167.03 (C). Infrared spectra (IR, ν, cm-1): 3344, 2970, 2887, 2660, 2544, 1709, 1673, 1610, 1592, 1526, 1511, 1411, 1311, 1282, 1221, 1052, 850, 736. reversed-phase high performance liquid chromatography (RP-HPLC) retention time: 26.6 Minutes. Mass spectrometry (FD-MS): m/z (%) = 359.1 (100), 360.1 (17.4); calculated value [C22H17NO4] = 359.1.

28920-43-6
553 suppliers
$5.00/5g

150-13-0
947 suppliers
$5.00/25g

185116-43-2
130 suppliers
$8.00/1g
Yield: 94%
Reaction Conditions:
for 24 h;Inert atmosphere;
Steps:
N-Fmoc-p-amino benzoic acid
p-Aminobenzoic acid (10 g, 73 mmol) was dissolved in dry NMP (50 ml) under an inert atmosphere followed by the dropwise addition of Fmoc-Cl (18.9 g, 73 mmol) in dry NMP (50 ml). After 24 hrs, the reaction mixture was poured slowly into 400 ml water. The colorless precipitate was collected by filtration, washed with water and dried in vacuum at 120 ºC to give N-Fmoc-p-amino benzoic acid (24.8 g, 94%). mp: 215 ºC (dec.) 1H-NMR: δ (300 MHz, DMSO-d6) 4.33 (t, 3J = 6.25 Hz, 1 H); 4.54 (d, 3J = 6.25 Hz, 2 H); 7.33-7.45 (m, 4 H); 7.57 (d, 3J = 7.35 Hz, 2 H); 7.76 (d, 3J = 7.35 Hz, 2 H); 7.89 (m, 4 H), 10.08 (s, 1H); 12.69 (s, 1H). 13C-NMR and DEPT: δ (300 MHz, DMSO-d6) 46.61 (+); 65.83 (-); 117.5 (+); 120.22 (+); 124.48; 125.14 (+); 127.17 (+); 127.74 (+); 130.48 (+); 140.86; 143.31; 143.74; 153.31; 167.03. IR ν (cm--1): 3344, 2970, 2887, 2660, 2544, 1709, 1673, 1610, 1592, 1526, 1511, 1411, 1311, 1282, 1221, 1052, 850, 736. RP-HPLC (min): 26.6 min. M (FD): m/z (%) = 359.1 (100); 360.1 (17.4); calc.[C22H17NO4] = 359.1
References:
Seyler, Helga;Kilbinger, Andreas [Tetrahedron Letters,2013,vol. 54,# 8,p. 753 - 756] Location in patent:supporting information
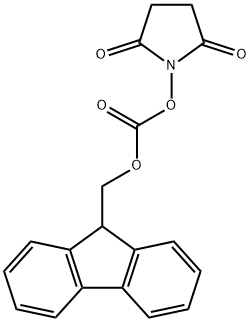
82911-69-1
668 suppliers
$7.00/25g

150-13-0
947 suppliers
$5.00/25g

185116-43-2
130 suppliers
$8.00/1g