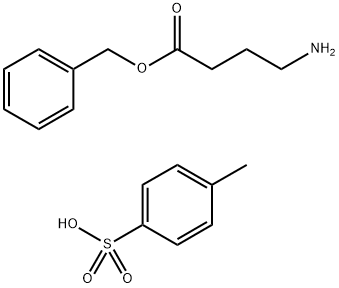
H-GAMMA-ABU-OBZL P-TOSYLATE synthesis
- Product Name:H-GAMMA-ABU-OBZL P-TOSYLATE
- CAS Number:26727-22-0
- Molecular formula:C18H23NO5S
- Molecular Weight:365.44
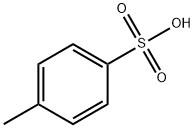
104-15-4

100-51-6
56-12-2

26727-22-0
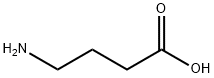
A suspension of γ-aminobutyric acid (GABA) (3.50 g, 0.034 mol), benzyl alcohol (7.30 g, 7 mL, 0.068 mol), and p-toluenesulfonic acid monohydrate (PTSA) (7.10 g, 0.037 mol) was heated to reflux for 5 hr in toluene (200 mL), and the resulting water was removed by azeotropic distillation. Upon completion of the reaction, the mixture was concentrated to one-third of the original volume and the product was subsequently precipitated by the addition of ether (100 mL). The precipitate was collected by filtration, dissolved in methanol (60 mL) and reprecipitated by adding ether (100 mL) again. After filtration, it was dried to obtain 4-methylbenzenesulfonate, 4-aminobutyric acid benzyl ester (12.30 g, 99% yield) in the form of white crystals. The melting point of the product was 106.2-106.7 °C (determined in ether); Infrared spectrum (ATR): 3100, 3039, 2942, 1732, 1642, 1532, 1188, 1125 cm^-1; ^1H NMR (200 MHz, CD3OD): δ = 7.71 (d, J = 8.0 Hz, 2H, HPTSA), and 7.37-7.30 (m, 5H, HCAr), 7.20 (d, J=8.4 Hz, 2H, HPTSA), 5.11 (s, 2H, H2CBn), 2.95 (t, J=7.5 Hz, 2H, H2C(4)GABA), 2.47 (t, J=7.3 Hz, 2H, H2C(2)GABA), 2.33 (s 3H, H3CPTSA), 1.92 (quint, J=7.4 Hz, 2H, H2C(3)GABA) ppm; ^13C NMR (50 MHz, CD3OD): δ=173.83 (CO2Bn), 143.35, 141.82, 137.43, 129.91, 129.59, 129.30, 126.91 (CAr), 67.44 (CH2Bn), 40.00 (CH2(4)GABA), 31.59 (CH2(2)GABA), 23.65 (CH2(3)GABA), 21.30 (CH3PTSA) ppm; Mass Spectra (ESI): calculated values of C11H16NO2 ([M+H]+): 194.1181, measured value: 194.1167.

104-15-4
765 suppliers
$133.00/500g

100-51-6
1434 suppliers
$5.00/100g

56-12-2
917 suppliers
$5.00/100mg

26727-22-0
64 suppliers
$23.30/5G
Yield:26727-22-0 99%
Reaction Conditions:
in toluene; for 5 h;Reflux;
Steps:
Compound 2.
A suspension of γ-aminobutanoic acid (GABA) 1 (3.50 g, 0.034 mol), benzyl alcohol (7.30 g, 7 mL,0.068 mmol) and p-toluenesulfonic acid monohydrate (PTSA) (7.10 g, 0.037 mol) in PhMe (200 mL) was heated toreflux for 5 h with azeotropic removal of water. The reaction mixture was concentrated to a one third of the volumeand the product precipitated by addition of Et2O (100 mL). The precipitate was filtered, dissolved in CH3OH (60 mL)and again precipitated by addition of Et2O (100 ml), giving after filtration and drying the benzyl ester 2 (12.30 g,99%) as white crystals. M.p. 106.2-106.7 C (Et2O); IR(ATR): 3100, 3039, 2942, 1732, 1642, 1532, 1188, 1125 cm-1;1H NMR (200 MHz, CD3OD): δ=7.71 (d, J=8.0 Hz, 2H, HPTSA), 7.37-7.30 (m, 5H, HCAr), 7.20 (d, J=8.4 Hz, 2H,HPTSA), 5.11 (s, 2H, H2CBn), 2.95 (t, J=7.5 Hz, 2H, H2C(4)GABA), 2.47 (t, J=7.3 Hz, 2H, H2C(2)GABA), 2.33 (s, 3H,H3CPTSA), 1.92 (quint, J=7.4 Hz, 2H, H2C(3)GABA) ppm; 13C NMR (50 MHz, CD3OD): δ=173.83 (CO2Bn), 143.35,141.82, 137.43, 129.91, 129.59, 129.30, 126.91 (CAr), 67.44 (CH2Bn), 40.00 (CH2(4)GABA), 31.59 (CH2(2)GABA), 23.65(CH2(3)GABA), 21.30 (CH3PTSA) ppm; MS(ESI): Calcd for C11H16NO2 (M+H)+: 194.1181, found: 194.1167.
References:
Kop, Tatjana J.;?or?evi?, Jelena;Bjelakovi?, Mira S.;Mili?, Dragana R. [Tetrahedron,2017,vol. 73,# 50,p. 7073 - 7078]

100-51-6
1434 suppliers
$5.00/100g

56-12-2
917 suppliers
$5.00/100mg

26727-22-0
64 suppliers
$23.30/5G