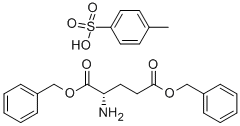
L-Glutamic acid dibenzyl ester tosylate synthesis
- Product Name:L-Glutamic acid dibenzyl ester tosylate
- CAS Number:2791-84-6
- Molecular formula:C26H29NO7S
- Molecular Weight:499.58
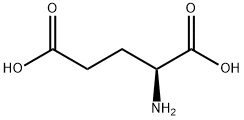
56-86-0
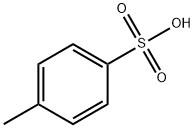
104-15-4

100-51-6

2791-84-6
General procedure for the synthesis of L-glutamic acid bisbenzyl ester p-toluenesulfonate from L-glutamic acid, 4-methylphenylsulfonic acid and benzyl alcohol: 29.4 g (0.2 mol) of L-(+)-glutamic acid, 40 g (0.23 mol) of p-toluenesulfonic acid and 80 mL of benzyl alcohol were dissolved in 500 mL of toluene. 11 mL of water was separated by refluxing under nitrogen protection. Refluxing was continued for 3 hours, followed by evaporation and removal of 150 mL of liquid. The reaction solution was cooled to 50°C and then poured into a beaker containing 600 mL of petroleum ether and stirred for 1 hour. The precipitate was collected by filtration. The filter cake was dissolved in 280 mL of 95% ethanol by heating, and after the heating was stopped, the solution was cooled overnight. The precipitate was collected by filtration and dried under vacuum to give 61 g of L-(+)-glutamic acid bisbenzyl ester p-toluenesulfonate (Compound 1).

56-86-0
872 suppliers
$5.00/25g

98-59-9
625 suppliers
$9.00/5g

100-51-6
1433 suppliers
$5.00/100g

2791-84-6
161 suppliers
$7.00/5g
Yield: 81%
Reaction Conditions:
in cyclohexane for 24 h;Reflux;
Steps:
General procedure for synthesis of 1a-1c:
General procedure: p-TsCl catalysed benzyl esterification of mono- and di- carboxylic acid is described in Scheme 1. For L-phenylalanine, since it contains monocarboxylic group, half equivalents of benzyl alcoholand p-TsCl were taken than those used for dicarboxylicamino acids l-aspartic acid and l-glutamic acid. Reaction mixture contained 1 gm (6.8 mmol) l-glutamic acid, 1.56gm (8.2 mmol) p-TsCl, 4 ml (38.6 mmol) benzyl alcoholand 20 ml (excess) cyclohexane. The reaction mixture wasrefluxed overnight under continuous stirring and progressof reaction was monitored through TLC. On completion,the reaction mixture was cooled to room temperature andethyl acetate was added till complete precipitation of products1a-1c. The precipitate obtained was filtered, washedwith cold ethyl acetate and dried to yield benzyl-protectedp-toluenesulfonate salts of respective amino acids.
References:
Hegde, Namita;Juvale, Kapil;Prabhakar, Bala [International Journal of Peptide Research and Therapeutics,2020,vol. 26,# 4,p. 2129 - 2135]

56-86-0
872 suppliers
$5.00/25g

104-15-4
764 suppliers
$133.00/500g

100-51-6
1433 suppliers
$5.00/100g

2791-84-6
161 suppliers
$7.00/5g