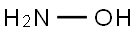
HYDROXYLAMINE synthesis
- Product Name:HYDROXYLAMINE
- CAS Number:7803-49-8
- Molecular formula:H3NO
- Molecular Weight:33.03
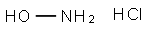
5470-11-1
814 suppliers
$10.00/5G
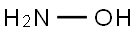
7803-49-8
233 suppliers
$14.00/5mL
Yield:-
Reaction Conditions:
with sodium hydroxide in methanol at 5 - 30; for 0.5 h;
Steps:
1.1.c
A solution of sodium hydroxide (3. 31 g, 55.2 mmol) in methanol (30 mL) was added to a hot solution of hydroxylamine hydrochloride (3.83 g, 55.2 mmol) in methanol (30 mL). The mixture was kept under stirring at 25-30 °C for 30 minutes and then cooled to 5-10 °C. The precipitate was removed by filtration. This freshly prepared solution of the hydroxylamine was added to 2- 3-amino-3- [4- (2-methoxymethyl-quinolin-4-ylmethoxy)-phenyl]-2-oxo- pyrrolidin-l-yl}-4-methyl-pentanoic acid methyl ester (1.4 g, 2.76 mmol) obtained above in methanol (10 mL) at 5-10 °C. The mixture was stirred at 25-30 °C for 2 h. and acidified to pH 6.0-6. 5 with 1 N HC1. The hydroxamic acid was precipitated out. The desired 2- {3-amino-3- [4- (2-methoxymethyl-quinohn-4-ylmethoxy)-phenyl]-2-oxo-pyrrolidin-l-yl}-4-methyl- pentanoic acid hydroxyamide was collected by filtration, washed with water (2 x 10 mL) and dried under vacuum to give the compound of formula (1) (1.0 g, 69.5 %).
References:
WO2005/77937,2005,A1 Location in patent:Page/Page column 20
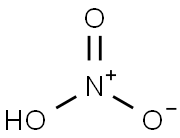
7697-37-2
0 suppliers
$13.00/25g
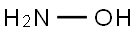
7803-49-8
233 suppliers
$14.00/5mL
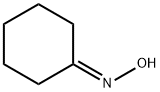
100-64-1
299 suppliers
$5.00/25g
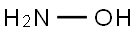
7803-49-8
233 suppliers
$14.00/5mL
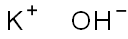
1310-58-3
738 suppliers
$10.00/1ea
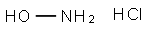
5470-11-1
814 suppliers
$10.00/5G
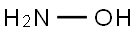
7803-49-8
233 suppliers
$14.00/5mL
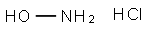
5470-11-1
814 suppliers
$10.00/5G
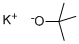
865-47-4
710 suppliers
$14.00/25g
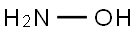
7803-49-8
233 suppliers
$14.00/5mL