Hypochlorous acid, methoxymethyl ester (9CI) synthesis
- Product Name:Hypochlorous acid, methoxymethyl ester (9CI)
- CAS Number:150587-19-2
- Molecular formula:C2H5ClO2
- Molecular Weight:96.51
Yield:120040-74-6 100%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 0 - 25;
Steps:
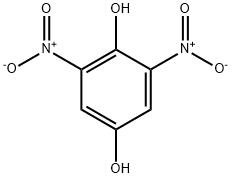
2,5-Bis(methoxymethoxy)-1,3-dinitrobenzene (6)5
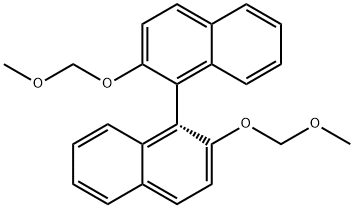
The current procedure was adapted from Kelly et al.5 with modification. To a solution 5 (1.18 g, 5.9 mmol) in anhyd THF at 0 °C was added DIPEA (3.1 mL, 18.1 mmol) followed by MOM-Cl (1.7 mL, 22.1 mmol). The solution was allowed to warm to r.t. and stirred for 3 h. The reaction mixture was diluted with EtOAc (200 mL) and washed with deionized H2O (2 × 200 mL). The organic layer was dried (anhyd Na2SO4), filtered, and concentrated to give 6 (1.7 g, 5.9 mmol, quant.) as a yellow oil that was used directly without further purification; Rf = 0.86 (hexane/EtOAc, 1:1). 1H NMR (400 MHz, CDCl3): δ = 7.68 (s, 2 H), 5.24 (s, 2 H), 5.14 (s, 2 H), 3.50 (s, 3 H), 3.49 (s, 3 H). 13C NMR (100 MHz, CDCl3): δ = 153.0, 146.6, 138.4, 116.6, 103.1, 95.3, 58.4, 56.8. MS (ESI): m/z [M + Na]+ calcd for C10H12N2O8Na+: 311.0; found: 311.0.
References:
Prior, Allan M.;Sun, Dianqing [Synthesis,2018,vol. 50,# 4,p. 859 - 871]
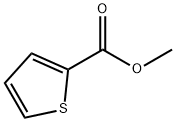
5380-42-7
177 suppliers
$6.00/1g

150587-19-2
0 suppliers
inquiry

7353-89-1
36 suppliers
$201.47/1gm:
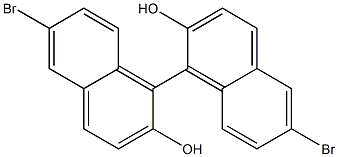
79082-80-7
8 suppliers
inquiry

150587-19-2
0 suppliers
inquiry
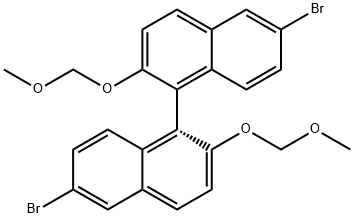
179866-74-1
65 suppliers
$23.00/250mg