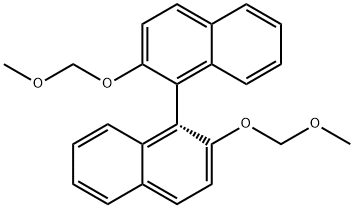
(R)-(+)-2,2'-BIS(METHOXYMETHOXY)-1,1'-BINAPHTHYL synthesis
- Product Name:(R)-(+)-2,2'-BIS(METHOXYMETHOXY)-1,1'-BINAPHTHYL
- CAS Number:173831-50-0
- Molecular formula:C24H22O4
- Molecular Weight:374.43
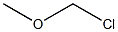
107-30-2
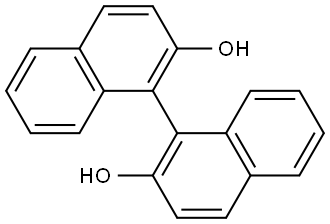
602-09-5

173831-50-0
Sodium hydride (NaH, 1.92 g, 80 mmol) was slowly added to N,N-dimethylformamide (DMF, 30 mL) under ice bath conditions. Subsequently, a tetrahydrofuran (THF, 50 mL) solution of 1,1'-bi-2-naphthol (BINOL, 10 g, 34 mmol) was added slowly dropwise to the above mixture for 20 min. after 30 min, chloromethyl methyl ether (6.4 g, 80 mmol) was added slowly dropwise for the same period of time. The reaction process was monitored by thin layer chromatography (TLC). The reaction mixture was extracted with chloroform and water after stirring for 1 h. The organic layer was separated to obtain the crude product. The crude product was further purified by silica gel column chromatography with petroleum ether/ethyl acetate (5:1, v/v) as eluent. Finally, (R)-2,2'-bis(methoxymethoxy)-1,1'-binaphthyl was obtained in 90% yield. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.95-7.93 (d, J = 9.6 Hz, 2H), 7.87-7.85 (d, J = 8.4 Hz, 2H), 7.58-7.56 (d, J = 9.6 Hz, 2H), 7.35-7.31 (m, 2H), 7.23-7.14 (m, 4H ), 5.08-4.96 (q, J = 6.8 Hz, 4H), 3.13 (s, 6H).
135-19-3
762 suppliers
$9.00/1g

173831-50-0
90 suppliers
$11.00/1g
Yield:-
Steps:
Multi-step reaction with 2 steps
1.1: iron(III) chloride hexahydrate / water / 3 h / 20 - 50 °C / Inert atmosphere
2.1: sodium hydride / mineral oil; tetrahydrofuran / 1 h / 0 °C / Inert atmosphere
2.2: 4.5 h / 0 - 20 °C / Inert atmosphere
References:
Lv, Na;Xie, Meilan;Gu, Weibing;Ruan, Huanyang;Qiu, Song;Zhou, Chunshan;Cui, Zheng [Organic Letters,2013,vol. 15,# 10,p. 2382 - 2385]
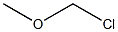
107-30-2
0 suppliers
$110.00/2.5g

602-09-5
349 suppliers
$6.00/5g

173831-50-0
90 suppliers
$11.00/1g
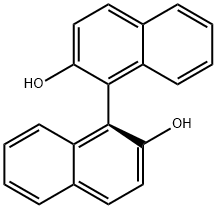
18531-99-2
406 suppliers
$10.00/250mg
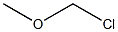
107-30-2
0 suppliers
$110.00/2.5g

173831-50-0
90 suppliers
$11.00/1g
