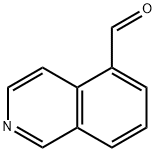
ISOQUINOLINE-5-CARBALDEHYDE synthesis
- Product Name:ISOQUINOLINE-5-CARBALDEHYDE
- CAS Number:80278-67-7
- Molecular formula:C10H7NO
- Molecular Weight:157.17
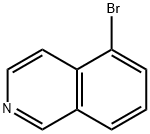
34784-04-8

68-12-2

80278-67-7
To a solvent mixture of ether (80 mL) and tetrahydrofuran (80 mL), n-butyllithium (19.3 mL, 2.5 M hexane solution, 48 mmol) was slowly added dropwise at -78 °C. Subsequently, a solution of 5-bromoisoquinoline (5.0 g, 24 mmol) in tetrahydrofuran (10 mL) was added dropwise. The reaction mixture was stirred at -78 °C and under argon protection for 30 min. Referring to the method of Pearson et al. in Journal of Heterocyclic Chemistry, Vol. 6, No. 2, pp. 243-245 (1969), a tetrahydrofuran (10 mL) solution of N,N-dimethylformamide (3.30 g, 45 mmol) was rapidly added to the lithium isoquinolinium solution after it was cooled to -78°C. The mixture was continued to be stirred at -78 °C for 15 minutes. Subsequently, ethanol (20 mL) and saturated ammonium chloride solution were added. The resulting suspension was slowly warmed to room temperature. The organic and ether extracted layers were combined and dried with anhydrous sodium sulfate. Purification by silica gel column chromatography (SiO2-H, hexane solution of 50% ethyl acetate) and ethanol recrystallization afforded isoquinoline-5-carbaldehyde (2.4 g, 15 mmol, 64% yield) as a light yellow solid with a melting point of 114-116 °C.1H NMR (DMSO-d6) δ 10.40 (s, 1H), 9.44 (s, 1H), 8.85 (d 1H, J = 6.0 Hz), 8.69 (d, 1H, J = 6.0 Hz), 8.45 (m, 2H), 7.90 (t, 1H, J = 7.2 Hz); 13C NMR (DMSO-d6) δ 194.23, 153.5, 146.2, 140.2, 135.2, 132.6, 130.2, 128.6, 127.5, 117.2. Calculated elemental values (C10H7NO-0.05H2O): C, 75.99; H, 4.53; N, 8.86. Measured values: C, 75.98; H, 4.66; N, 8.68.
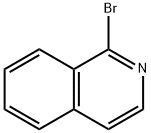
1532-71-4
206 suppliers
$9.00/100mg

80278-67-7
161 suppliers
$25.00/250mg
Yield:-
Reaction Conditions:
with n-butyllithium;ammonium chloride in tetrahydrofuran;ethanol;N,N-dimethyl-formamide
Steps:
2 5-Isoquinolinecarboxaldehyde
EXAMPLE 2 5-Isoquinolinecarboxaldehyde To a solution of n-butyllithium (19.3 mL of 2.5M in hexanes, 48 mmol) in a mixture of ether (80 mL) and THF (80 mL) at -78° C. was added dropwise a solution of bromoisoquinoline (5.0 g, 24 mmol) in THF (10 mL). The reaction mixture was stirred at -78° C. under argon for 30 minutes. Following the general procedures described by Pearson, et al., in J. Heterocycl. Chem., Vol. 6 (2), pp. 243-245 (199), a solution of DMF (3.30 g, 45 mmol) in THF (10 mL) was cooled to -78° C. and quickly added to the isoquinolyllithium solution. The mixture was stirred at -78° C. for 15 minute. Ethanol (20 mL) was added followed by saturated NH4Cl solution. The resulting suspension was warmed to room temperature. The organic layer, combined with the ether extraction layer, was dried over Na2SO4. A pale yellow solid (2.4 g, 15 mmol, 64% yield) was obtained from chromatography (SiO2 Type-H, 50% EtOAc in hexanes) and recrystallization (ethanol): mp 114-116° C.;
References:
Purdue Research Foundation US6645975, 2003, B1
16675-59-5
60 suppliers
$28.00/100mg

80278-67-7
161 suppliers
$25.00/250mg
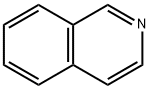
119-65-3
428 suppliers
$5.00/25g

80278-67-7
161 suppliers
$25.00/250mg
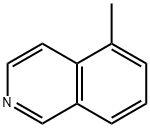
62882-01-3
23 suppliers
inquiry

80278-67-7
161 suppliers
$25.00/250mg
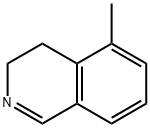
100643-54-7
0 suppliers
inquiry

80278-67-7
161 suppliers
$25.00/250mg