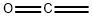
ketene synthesis
- Product Name:ketene
- CAS Number:463-51-4
- Molecular formula:C2H2O
- Molecular Weight:42.04

64-17-5
825 suppliers
$10.00/50g
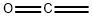
463-51-4
14 suppliers
inquiry

75-07-0
428 suppliers
$14.00/5mL

64-19-7
1626 suppliers
$10.00/25ML

141-78-6
685 suppliers
$20.50/250ml
Yield:-
Reaction Conditions:
with oxygen at -133.16; under 4E-10 Torr;
Steps:
For the reaction studies, the pre-conditioned npAu catalyst was exposed to molecular oxygen at 300 K by backfilling the vacuum chamber via a leak valve, and all exposures reported here are in units of Langmuir (1L = 10-6 Torr s). The relative oxygen coverage (in percent of the saturation coverage) was determined by comparison with the oxygen saturation calibration curve (see below). Ethanol and 1-butanol were introduced to the catalyst surface at the temperatures specified (typically below 180 K) by monitoring the ambient pressure in the chamber. Surface concentrations of Oads used were relatively low (10 % of saturation) in order to minimize over oxidation of the alcohols. It is not possible to determine the absolute coverage of Oads on npAu becausethe active surface area was not known. A typical experiment consisted of oxygen exposure to the catalyst surface at 300 K followed by cooling of the sample to180-140 K before dosing the alcohol. A trace amount ofwater condensed on the surface during cooling (less than1 % of the amount observed in a typical alcohol oxidationreaction). The catalyst was then heated, and the reactionmonitored by temperature programmed reaction spectroscopy (TPRS) according to well-established protocols. The heating rate for all temperature programmed reactions was nearly constant at *3 ± 0.5 K s-1. The reaction products were identified by quantitative mass spectrometry (Hiden HAL/3F) using fragmentation patterns obtained from authentic samples (see Supporting Information).
References:
Stowers, Kara J.;Madix, Robert J.;Biener, Monika M.;Biener, Juergen;Friend, Cynthia M. [Catalysis Letters,2015,vol. 145,# 6,p. 1217 - 1223]
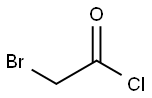
22118-09-8
182 suppliers
$21.21/10gm:
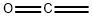
463-51-4
14 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML
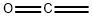
463-51-4
14 suppliers
inquiry

64-19-7
1626 suppliers
$10.00/25ML
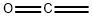
463-51-4
14 suppliers
inquiry

74-86-2
76 suppliers
inquiry
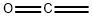
463-51-4
14 suppliers
inquiry