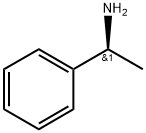
L-1-Phenylethylamine synthesis
- Product Name:L-1-Phenylethylamine
- CAS Number:2627-86-3
- Molecular formula:C8H11N
- Molecular Weight:121.18
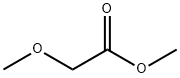
6290-49-9
206 suppliers
$21.00/25g
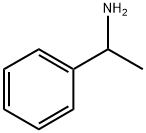
618-36-0
297 suppliers
$10.00/5mL

2627-86-3
540 suppliers
$10.60/1gm:
![Acetamide, 2-methoxy-N-[(1R)-1-phenylethyl]-](/CAS/20180529/GIF/162929-44-4.gif)
162929-44-4
2 suppliers
inquiry
Yield:> 99 % ee
Reaction Conditions:
with 5% palladium on barium sulphate;Lipase B from Candida antarctica immobilized on SiO2 nanoparticles functionalized with 1 % Pd and (3-aminopropyl)triethoxysilane;ammonium formate;sodium carbonate in toluene at 70; for 17 h;Molecular sieve;Enzymatic reaction;
Steps:
Dynamic kinetic resolution
General procedure: 0.3 mmol of α-methylbenzylamine, 2 eq. of methyl methoxyacetate (acyl donor), the biocatalyst (94 mg·mmol-1 of substrate), molecular sieves (30 mol%, 375 mg), Na2CO3 (12 mg), 15 eq. of ammonium formate (as hydrogen source) and toluene (3 mL) were added in closed 4 mL vials equipped with a gas bubbler and heated at 70 °C in silicon carbide plates for 17 h. The same experiment was performed with commercial N435 using 5 % of Pd supported on BaSO4 as a racemization agent. Conversions and enantiomeric excesses were determined by GC-FID (Shimadzu CG2010 - chiral capillary column CP-Chirasil-Dex CB): Injector and detector temperatures were set to 220 °C. The oven temperature set to 105 °C, and held for 9 min. Then the temperature was raised to 180 °C at rate of 140 °C/min. The final temperature was held isothermally for 10 min.
References:
de Souza, Stefania P.;Le?o, Raquel A.C.;Bassut, Jonathan F.;Leal, Ivana C.R.;Wang, Shuai;Ding, Qiqi;Li, Yingying;Lam, Frank Leung-Yuk;de Souza, Rodrigo O.M.A.;Itabaiana Jr, Ivaldo [Tetrahedron Letters,2017,vol. 58,# 52,p. 4849 - 4854]
![2-Propanesulfinamide, 2-methyl-N-(1-phenylethylidene)-, [S(S)]-](/CAS/20210305/GIF/874291-45-9.gif)
874291-45-9
0 suppliers
inquiry

2627-86-3
540 suppliers
$10.60/1gm:
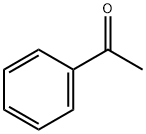
98-86-2
792 suppliers
$9.00/5g

2627-86-3
540 suppliers
$10.60/1gm:

98-86-2
792 suppliers
$9.00/5g

2627-86-3
540 suppliers
$10.60/1gm:
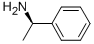
3886-69-9
567 suppliers
$14.00/25G
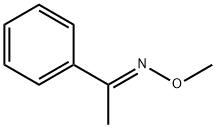
15754-20-8
0 suppliers
inquiry

2627-86-3
540 suppliers
$10.60/1gm: