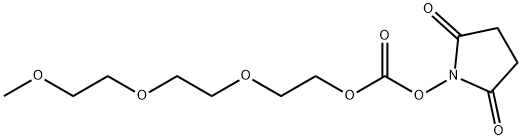
m-PEG3-NHS carbonate synthesis
- Product Name:m-PEG3-NHS carbonate
- CAS Number:477775-77-2
- Molecular formula:C12H19NO8
- Molecular Weight:305.28
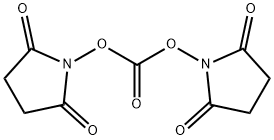
74124-79-1
476 suppliers
$5.00/5g

112-35-6
314 suppliers
$6.00/25g

477775-77-2
37 suppliers
$446.25/100MG
Yield:477775-77-2 75%
Reaction Conditions:
Stage #1: triethylene glucol monomethyl etherwith triethylamine in dichloromethane at 0; for 0.25 h;
Stage #2: di(succinimido) carbonate in dichloromethane at 20; for 18 h;
Steps:
m-PEG-3-SC-carbonate (15)
m-PEG-3-SC-carbonate (15) (0148) Into a 100 mL flask was placed mPEG3-OH (14) (2.0 g, 12.1 mmol) and anhydrous dichloromethane (25 mL). The clear solution was cooled to 0° C., and then triethylamine (1.86 mL, 13.4 mmol, 1.1 equivalents) was added slowly. The solution was stirred for 15 minutes at 0° C., and then was added to a second flask containing a suspension of DSC (3.1 g, 12.1 mmol) in dichloromethane (20 mL). The reaction mixture was allowed to equilibrate to room temperature. After approximately 18 hours, the light-yellow reaction mixture was diluted with dichloromethane (60 mL), transferred to a separatory funnel, and partitioned with deionized water (100 mL). The aqueous layer was extracted with dichloromethane (4×80 mL). The combined organics were washed with water, saturated sodium bicarbonate, and saturated sodium chloride. The dried organic layer was filtered, concentrated under reduced pressure and dried overnight under high vacuum, to give 2.79 g (75%) of mPEG3-SC-carbonate as a light yellow oil. 1H NMR (CDCl3) δ 4.40 (m, 2H), 3.80 (m, 2H), 3.70 (bs, 6H), 3.60 (m, 2H), 3.35 (s, 3H), 2.80 (s, 4H); LC/MS=306 (M+1).
References:
US2017/28077,2017,A1 Location in patent:Paragraph 0148
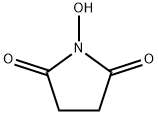
6066-82-6
805 suppliers
$5.00/10g
![Carbonochloridic acid, 2-[2-(2-methoxyethoxy)ethoxy]ethyl ester](/CAS/20200611/GIF/105292-71-5.gif)
105292-71-5
1 suppliers
inquiry

477775-77-2
37 suppliers
$446.25/100MG

112-35-6
314 suppliers
$6.00/25g

477775-77-2
37 suppliers
$446.25/100MG