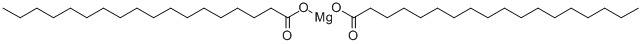
Magnesium stearate synthesis
- Product Name:Magnesium stearate
- CAS Number:557-04-0
- Molecular formula:C36H70MgO4
- Molecular Weight:591.24
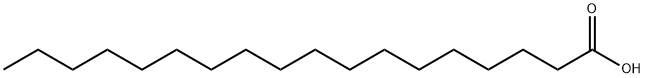
57-11-4
1307 suppliers
$6.00/100g
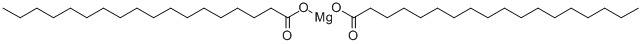
557-04-0
999 suppliers
$5.00/25g
Yield:557-04-0 95.23%
Reaction Conditions:
with magnesium oxide;dihydrogen peroxide;magnesium oxide at 100 - 130; for 0.75 h;Temperature;
Steps:
1
S2: The stearic acid in S1 was added to the magnesium stearate reactor, the temperature was kept at 100°C, while stirring added magnesium oxide 4% of the mass of the Glycerol Tristearate. After the feed was completed, the temperature of the material rose to 110°C. then added catalyst B 5% of the mass of the Glycerol Tristearate, and stirred the reaction for 25min; wherein, Catalyst B is a mixture of hydrogen peroxide and magnesium peroxide, in terms of mass ratio, hydrogen peroxide: magnesium peroxide was 5:1; S3: added magnesium oxide 1.5% of the mass of the Glycerol Tristearate and catalyst B 1% of the mass of the Glycerol Tristearate, then carried out the reaction at a temperature of 120°C, stopped the stirring, and the material foam was reduced to an equilibrium point then additionally added catalyst B 1% of the mass of the Glycerol Tristearate, and reacted at a temperature of 130 °C for 20 min; S4: while stirring added sodium hydroxide 0.1% of the mass of the Glycerol Tristearate, and the reaction temperature was 10min; S5: Open the vacuum pump, adjust the vacuum to -0.08MPa, dehydration, under constant temperature and pressure conditions carried out stable reaction for 60min, the reaction was terminated. After sampling test, qualification, it was dusted and packaged into the warehouse to obtain magnesium stearate, its yield is 95.23%.
References:
CN105949049,2016,A Location in patent:Paragraph 0033-0037
69-65-8
756 suppliers
$5.00/25g
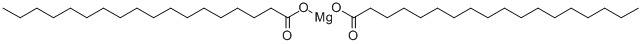
557-04-0
999 suppliers
$5.00/25g
7631-86-9
692 suppliers
$14.30/50g
151-21-3
1091 suppliers
$6.00/25g
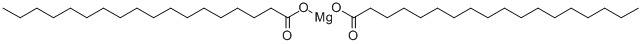
557-04-0
999 suppliers
$5.00/25g