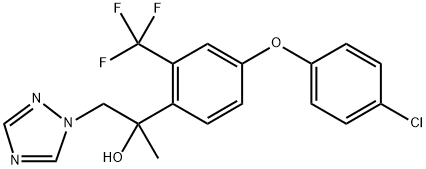
Mefentrifluconazole synthesis
- Product Name:Mefentrifluconazole
- CAS Number:1417782-03-6
- Molecular formula:C18H15ClF3N3O2
- Molecular Weight:397.78
Yield:1417782-03-6 92%
Reaction Conditions:
with sodium hydroxide in N,N-dimethyl-formamide at 115; for 12 h;
Steps:
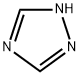
Preparation of 2-[4-(4-chlorophenoxy)-2-(trifluoromethyl)phenyl]-1 -(1 ,2,4-triazol-1 -yl)propan-2-ol
555.5 g (1 .69 mol) of 2-[4-(4-ch lorophenoxy)-2-(trifl uoromethyl)phenyl]-2-methyl-oxi rane, 152.0 g(2.2 mol) of 1H-[1,2,4]-triazole, 34.0 g (0.85 mol) of NaOH (flakes) and 1381 g of DMF werecharged to a 2.5 I laboratory vessel at room temperature. The mixture was heated to 115°C over12 h for full conversion of the oxirane starting material (yield: 92 % in solution for desired isomer).Afterwards almost the complete DMF (> 95%) was removed by vacuum distillation from the reaction mixture. Salts and the remaining DMF were separated from the product by extraction with 1690 g toluene and 1039 g water at 80°C. Finally 1318 g (78%) of the toluene was removed from the product by concentrating the organic phase under vacuum.DMF for crystallization was added to the product solution in toluene at 85°C. The DMF amount iscompiled in the table below. The percentages relate to the amount of DMF contained in theobtained mixture, relative to the total weight of the mixture obtained after the respective amount of DMF has been added. Afterwards the solution in toluene I DMF was cooled to approx. 70°C, seeded with the title product and stirred over 0.5 h. The suspension was slowly cooled down to 0°C over 8 h for crystallization of the product. The product was separated by centrifugation fromthe mother liquor and dried in a vacuum oven at 80°C I 50 mbar.
References:
BASF AGRO B.V.;GEBHARDT, Joachim;SAELINGER, Daniel;EHRESMANN, Manfred;GOETZ, Roland WO2017/102905, 2017, A1 Location in patent:Page/Page column 8
106-48-9
498 suppliers
$10.00/5 g

1417782-03-6
40 suppliers
inquiry
40161-55-5
334 suppliers
$10.00/1g

1417782-03-6
40 suppliers
inquiry
1417782-30-9
5 suppliers
inquiry

1417782-03-6
40 suppliers
inquiry
![1-[4-(4-Chloro-phenoxy)-2-trifluoromethyl-phenyl]ethanone](/CAS/20180531/GIF/1417782-28-5.gif)
1417782-28-5
25 suppliers
inquiry

1417782-03-6
40 suppliers
inquiry
![2-[4-(4-Chloro-phenoxy)-2-trifluoromethyl-phenyl]-2-methyl-oxirane](/CAS/20180601/GIF/1417782-29-6.gif)