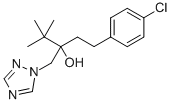
Tebuconazole synthesis
- Product Name:Tebuconazole
- CAS Number:107534-96-3
- Molecular formula:C16H22ClN3O
- Molecular Weight:307.82
![4H-1,2,4-Triazole-4-ethanol, α-[2-(4-chlorophenyl)ethyl]-α-(1,1-dimethylethyl)-](/CAS/20210305/GIF/97821-63-1.gif)
97821-63-1

107534-96-3
To a 250 mL three-necked flask was added 120 g of N,N-dimethylformamide, followed by 40 g of pentaconazole isomer, 2.0 g of potassium hydroxide, and 1.0 g of 3-bromomethyl-1-(4-chlorophenyl)-4,4-dimethyl-3-propanol.The reaction mixture was heated to 150 °C and maintained at this temperature for 4 hours. Sampling and analysis during the reaction showed that the purity of tebuconazole was 99.8% and the isomer content was 0.2%. Upon completion of the reaction, the target product tebuconazole was obtained by removing the solvent, followed by slurry treatment with water and toluene, and finally filtered to give 38.6 g of the target product tebuconazole in 96.5% yield and 98.5% product purity.
![4H-1,2,4-Triazole-4-ethanol, α-[2-(4-chlorophenyl)ethyl]-α-(1,1-dimethylethyl)-](/CAS/20210305/GIF/97821-63-1.gif)
97821-63-1
1 suppliers
inquiry

107534-96-3
432 suppliers
$32.00/100mg
Yield:107534-96-3 96.5%
Reaction Conditions:
with 3-(bromomethyl)-1-(4-chlorophenyl)-4,4-dimethylpentan-3-ol;potassium hydroxide in N,N-dimethyl-formamide at 150; for 4 h;Reagent/catalyst;Solvent;Temperature;
Steps:
1
250 ml three-mouth bottle adding N, N - dimethyl formamide 120g, and add tebuconazole isomer 40g, potassium hydroxide 2.0g, 3 - bromomethyl -1 - (4 - chlorophenyl) - 4, 4 - dimethyl -3 - propanol 1.0g. The temperature to 150 °C reaction 4h. Sampling, the measured tebuconazole 99.8%, isomer 0.2%. After the desolvation, water and toluene after pulping, filtered to obtain product tebuconazole 38.6g, yield 96.5%, content of 98.5%.
References:
CN106699675,2017,A Location in patent:Paragraph 0024-0026
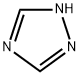
288-88-0
806 suppliers
$10.00/5g
![2-[2-(4-Chlorophenyl)ethyl]-2-(1,1-dimethylethyl)-oxirane](/CAS/GIF/80443-63-6.gif)
80443-63-6
113 suppliers
$28.00/5g

107534-96-3
432 suppliers
$32.00/100mg
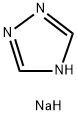
41253-21-8
283 suppliers
$5.00/5g
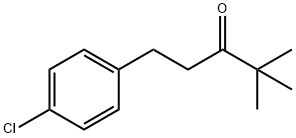
66346-01-8
162 suppliers
$50.00/1g

107534-96-3
432 suppliers
$32.00/100mg