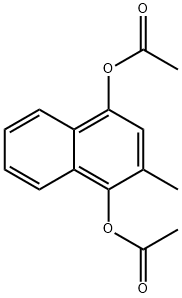
Menadiol diacetate synthesis
- Product Name:Menadiol diacetate
- CAS Number:573-20-6
- Molecular formula:C15H14O4
- Molecular Weight:258.27
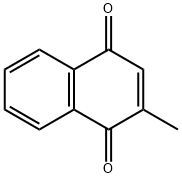
58-27-5

108-24-7

573-20-6
General procedure for the synthesis of vitamin K4 from 2-methyl-1,4-naphthoquinone (20.0 g) and acetic anhydride (29.65 g): 2-methyl-1,4-naphthoquinone was first dissolved in ethyl acetate (200 g). Subsequently, acetic anhydride, 4-dimethylaminopyridine (DMAP, 1.42 g), and palladium/carbon catalyst (Pd/C, 0.6 g) were added to the solution. The reaction mixture was stirred at room temperature in a hydrogen atmosphere overnight. Upon completion of the reaction, the Pd/C catalyst was removed by filtration. The filtrate was washed with brine and the organic layer was separated. The organic layer was concentrated and recrystallized in a solvent mixture of water (60 g) and isopropanol (IPA, 80 g) to yield 28.0 g of vitamin K4 as white crystals (yield: 84.2%, HPLC purity: 99.8%). The structure of the compound was characterized by 1H-NMR with the following chemical shifts: δ 2.27 (s, 3H), 2.46 (s, 3H), 2.50 (s, 3H), 7.27 (s, 1H), 7.55-7.62 (m, 2H), 7.85-7.89 (d, 2H).
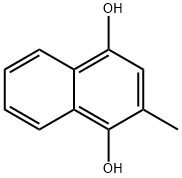
481-85-6
80 suppliers
$35.00/100mg

108-24-7
0 suppliers
$14.00/250ML

573-20-6
258 suppliers
$25.00/10g
Yield:573-20-6 90.2 %
Reaction Conditions:
with pyridine at 65 - 70;
Steps:
1.2; 2; 3.2
(2) the above0.45 g (0.006 mol) of pyridine is added to the 2-methyl-1,4-naphthalenediol ethyl acetate solution,17.1 g (0.167 mol) of acetic anhydride was added dropwise at 65-70° C. and aged at the same temperature for 3 hours. The organic layer was washed with city water and 5% sodium bicarbonate water,The organic layer was concentrated, and a mixed solution of isopropanol (IPA) and city water was added to the residue. The resulting slurry was filtered and dried under reduced pressure to give 12.0 g of colorless crystalline powder of 1,4-diacetoxy-2-methylnaphthalene.(0.046 mol) was obtained.HPLC purity is 99.96 Area%,The yield from bisulfite trihydrate of 2-methylnaphthoquinone was 90.2%.
References:
JP2023/16443,2023,A Location in patent:Paragraph 0023-0025

58-27-5
580 suppliers
$5.00/250mg

108-24-7
0 suppliers
$14.00/250ML

573-20-6
258 suppliers
$25.00/10g

58-27-5
580 suppliers
$5.00/250mg

64-19-7
1628 suppliers
$10.00/25ML

573-20-6
258 suppliers
$25.00/10g

67-56-1
790 suppliers
$9.00/25ml

58-27-5
580 suppliers
$5.00/250mg

108-24-7
0 suppliers
$14.00/250ML

573-20-6
258 suppliers
$25.00/10g
3526-72-5
1 suppliers
inquiry

58-27-5
580 suppliers
$5.00/250mg

573-20-6
258 suppliers
$25.00/10g