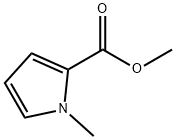
METHYL 1-METHYLPYRROLE-2-CARBOXYLATE synthesis
- Product Name:METHYL 1-METHYLPYRROLE-2-CARBOXYLATE
- CAS Number:37619-24-2
- Molecular formula:C7H9NO2
- Molecular Weight:139.15
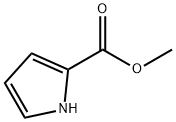
1193-62-0

74-88-4

37619-24-2
Methyl-1H-pyrrole-2-carboxylate (10 g, 79.9 mmol) was dissolved in anhydrous THF (200 mL) at 0 °C and NaH (3.52 g, 60% w/w, 87.9 mmol) was slowly added. The reaction mixture was stirred at 20 °C for 30 min before CH3I (13.62 g, 95.9 mmol) was added dropwise. The reaction was continued to be stirred at 20 °C for 17 hours. After completion of the reaction, the reaction was quenched by slowly pouring the mixture into aqueous NH4Cl (200 mL). The aqueous phase was extracted with EtOAc (200 mL x 2), the organic layers were combined and washed with brine (150 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: EtOAc/petroleum ether=1/20) to afford methyl 1-methyl-1H-pyrrole-2-carboxylate (9.88 g, 71 mmol, 89% yield) as a yellow oil. Mass spectrum (EI+, m/z): 140.1 [M+H]+.

67-56-1
790 suppliers
$7.29/5ml-f
6973-60-0
207 suppliers
$6.00/250mg

18107-18-1
211 suppliers
$33.00/5g

37619-24-2
112 suppliers
$10.00/500mg
Yield:-
Reaction Conditions:
at 20; for 1 h;
Steps:
26
To a solution of 1-methyl-2-pyrrolecarboxylic acid (1.03 g, 8.22 mmol) in MeOH (5 mL) and dioxane (5 mL) at room temperature, trimethylsilyldiazomethane (2M in ether, 5.0 mL, 10.0 mmol) was added. After being stirred at room temperature for 1 h, the mixture was concentrated in vacuo to give methyl 1-methyl-2-pyrrolecarboxylate as a volatile oil (1.14 g).
References:
Millennium Pharmaceuticals, Inc. US2007/259924, 2007, A1 Location in patent:Page/Page column 33

1193-62-0
228 suppliers
$5.00/1g

74-88-4
357 suppliers
$15.00/10g

37619-24-2
112 suppliers
$10.00/500mg

6973-60-0
207 suppliers
$6.00/250mg

74-88-4
357 suppliers
$15.00/10g

37619-24-2
112 suppliers
$10.00/500mg

1193-62-0
228 suppliers
$5.00/1g

616-38-6
722 suppliers
$5.00/5 g

37619-24-2
112 suppliers
$10.00/500mg

616-38-6
722 suppliers
$5.00/5 g
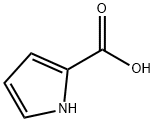
634-97-9
290 suppliers
$6.00/1g

37619-24-2
112 suppliers
$10.00/500mg