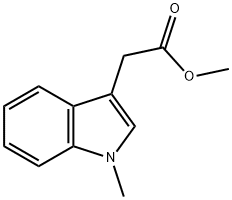
Methyl 2-(1-methyl-1H-indol-3-yl)acetate synthesis
- Product Name:Methyl 2-(1-methyl-1H-indol-3-yl)acetate
- CAS Number:58665-00-2
- Molecular formula:C12H13NO2
- Molecular Weight:203.24
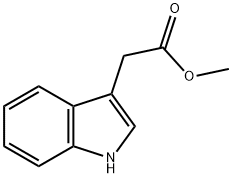
1912-33-0

74-88-4

58665-00-2
Methyl 2-(1H-indol-3-yl)acetate (200 mg, 1.1 mmol) was dissolved in N,N-dimethylformamide (3 mL). To this solution was added sodium hydride (60 mg, 60% dispersed in mineral oil, 1.5 mmol). Subsequently, iodomethane (223 mg, 1.58 mmol) was added and the reaction mixture was stirred at room temperature for 6 hours. The progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction solution was acidified to pH 3-4 with 6N hydrochloric acid, diluted with the addition of water (5 mL), and then extracted with ethyl acetate (5 mL × 3). The organic layers were combined, washed twice with saturated saline and dried over anhydrous sodium sulfate. After concentration under reduced pressure to remove the solvent, the residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate = 5:1) to afford methyl 2-(1-methyl-1H-indol-3-yl)acetate (140 mg, 65% yield).1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 7.9 Hz, 1H), 7.29 (d, J = 8.2 Hz, 1H), 7.23 (dd, J = 8.2, 7.9 Hz, 1H), 7.12 (dd, J = 8.2, 7.9 Hz, 1H), 7.03 (s, 1H), 3.77 (s, 3H), 3.75 (s, 2H), 3.69 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 172.6, 136.9, 127.7 136.9, 127.7, 121.7 (2C), 119.26, 118.9, 109.3, 106.8, 51.9, 32.7, 31.0.

67-56-1
790 suppliers
$9.00/25ml
1912-48-7
114 suppliers
$14.00/1g

58665-00-2
29 suppliers
$89.00/250mg
Yield:58665-00-2 100%
Reaction Conditions:
with hydrogenchloride for 1 h;Heating;
References:
Pour, Milan;Spulak, Marcel;Balsanek, Vojtech;Kunes, Jiri;Kubanova, Petra;Buchta, Vladimir [Bioorganic and Medicinal Chemistry,2003,vol. 11,# 13,p. 2843 - 2866]

1912-33-0
154 suppliers
$8.00/1g

74-88-4
357 suppliers
$15.00/10g

58665-00-2
29 suppliers
$89.00/250mg

1912-48-7
114 suppliers
$14.00/1g

18107-18-1
210 suppliers
$33.00/5g

58665-00-2
29 suppliers
$89.00/250mg
87-51-4
736 suppliers
$11.19/5G

616-38-6
722 suppliers
$5.00/5G

58665-00-2
29 suppliers
$89.00/250mg

87-51-4
736 suppliers
$11.19/5G

616-38-6
722 suppliers
$5.00/5G

1912-33-0
154 suppliers
$8.00/1g

58665-00-2
29 suppliers
$89.00/250mg