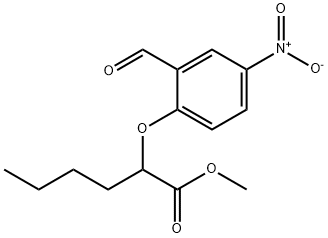
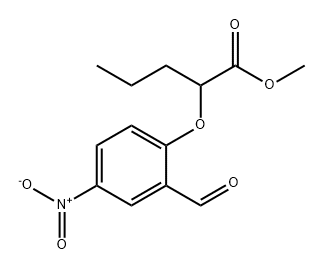
Methyl 2-(2-forMyl-4-nitrophenoxy)hexanoate synthesis
- Product Name:Methyl 2-(2-forMyl-4-nitrophenoxy)hexanoate
- CAS Number:335153-23-6
- Molecular formula:C14H17NO6
- Molecular Weight:295.29
138320-26-0
14 suppliers
inquiry

335153-23-6
25 suppliers
inquiry
Yield:335153-23-6 92.4%
Reaction Conditions:
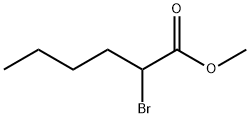
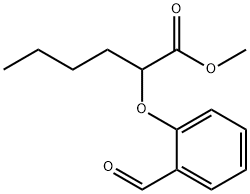
Stage #1: methyl 2-(2-formylphenoxy)hexanoatewith sulfuric acid at 5; for 0.25 h;
Stage #2: at 5; for 2 h;
Steps:
3 EXAMPLE 3, Preparation of methyl 2-(2-formyl-4-nitro-phenoxy)hexanoate
EXAMPLE 3 [00324] Preparation of methyl 2-(2-formyl-4-nitro-phenoxy)hexanoate [00325] Methyl 2-(2-formyl-4-nitro-phenoxy)-hexanoate can be prepared as follows: [00326] 123 g of 96% concentrated sulphuric acid was charged into a 250 ml four-neck reactor provided with a Teflon half moon paddle stirrer, a thermometer, a 50 ml dropping funnel, a cooling coil and a nitrogen inlet. [00327] The reaction medium was cooled to a temperature of close to 5° C. then 30 g (0.12 mole) of methyl 2-(2-formylphenoxy)-hexanoate was added at the same temperature. [00328] After stirring for 15 minutes, 15.9 g (0.126 mole) of nitrating mixture (50/50) was added over 2 hours, keeping the reaction medium close to 5° C., then 76.9 g of ice was added over 30 minutes, leading to an H2SO4 titre of 60%. [00329] The reaction mixture was filtered through a no 3 frit after stirring for 10 minutes. [00330] The crude product obtained was dissolved in 100 ml of dichloromethane and washed with twice 50 ml of water. [00331] The decanted organic phase was concentrated in a rotary evaporator at 20° C. to 70° C. in 20 mm of mercury (duration: 2 hours). [00332] 32.7 g of a beige yellow solid product was obtained, giving a yield of methyl 2-(2-formyl-4-nitro-phenoxy)-hexanoate of 92.4%, titrating at 96.7% by gas chromatography. [00333] It had the following NMR spectrum: [00334] 1H NMR (DMSO-d6): δ0.91 (t, 3H, CH3); 1.38 (m, 2H, CH2-CH3); 1.51 (m, 2H, CH2-CH2-CH3); 2.02 (m, 2H, CH2-CH); 5.24 (t, 1H, CH); 7.34 (d, J=9 Hz, 1H, ArH); 8.44 (d, J=2 Hz, 1H, ArH); 8.47 (dd, J=9 Hz, J=2 Hz, 1H, ArH); 10.42 (s, 1H, CHO); 13.45 (broad peak, 1H, COOH).
References:
US6855842,2005,B1 Location in patent:Page/Page column 16

847869-79-8
0 suppliers
inquiry

335153-23-6
25 suppliers
inquiry
847869-80-1
0 suppliers
inquiry

335153-23-6
25 suppliers
inquiry